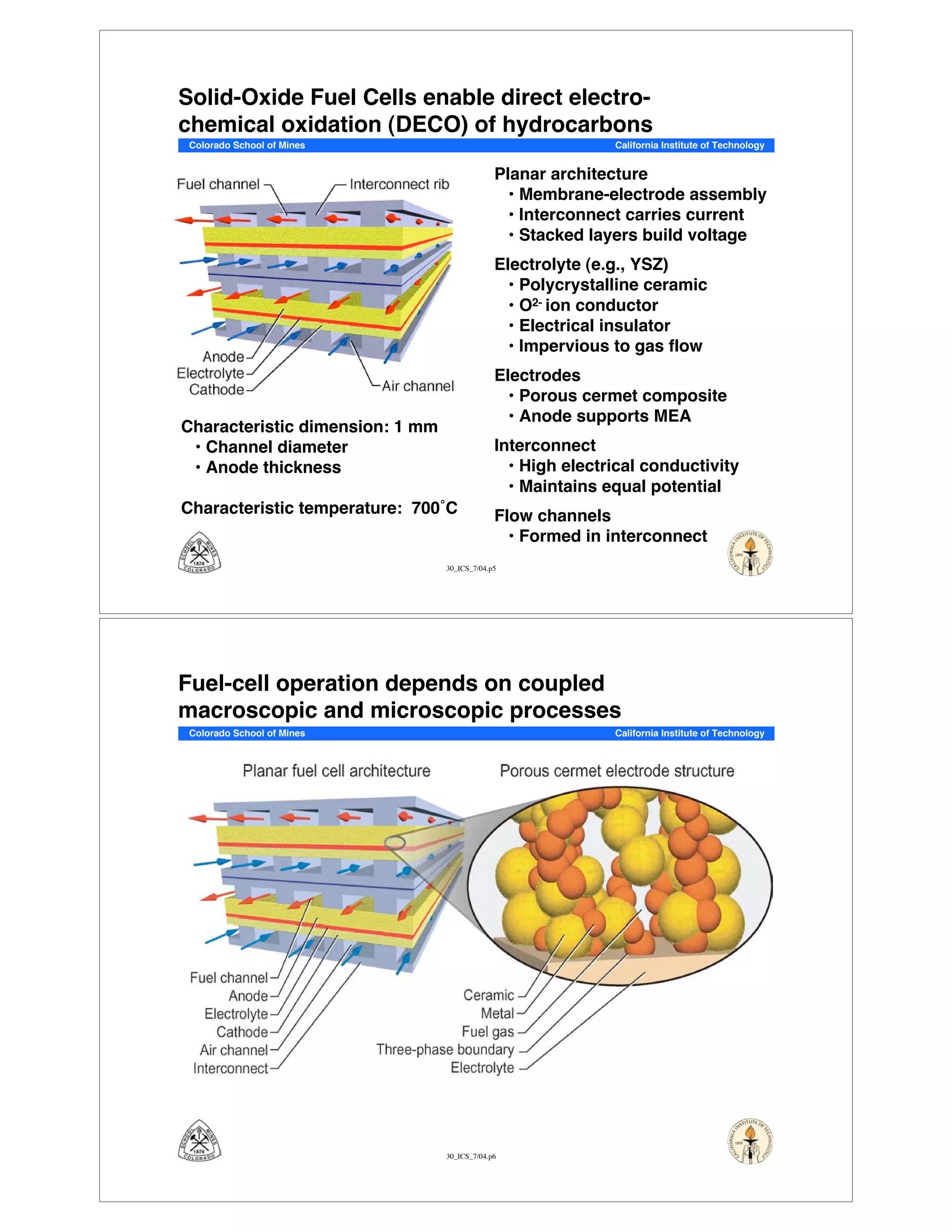
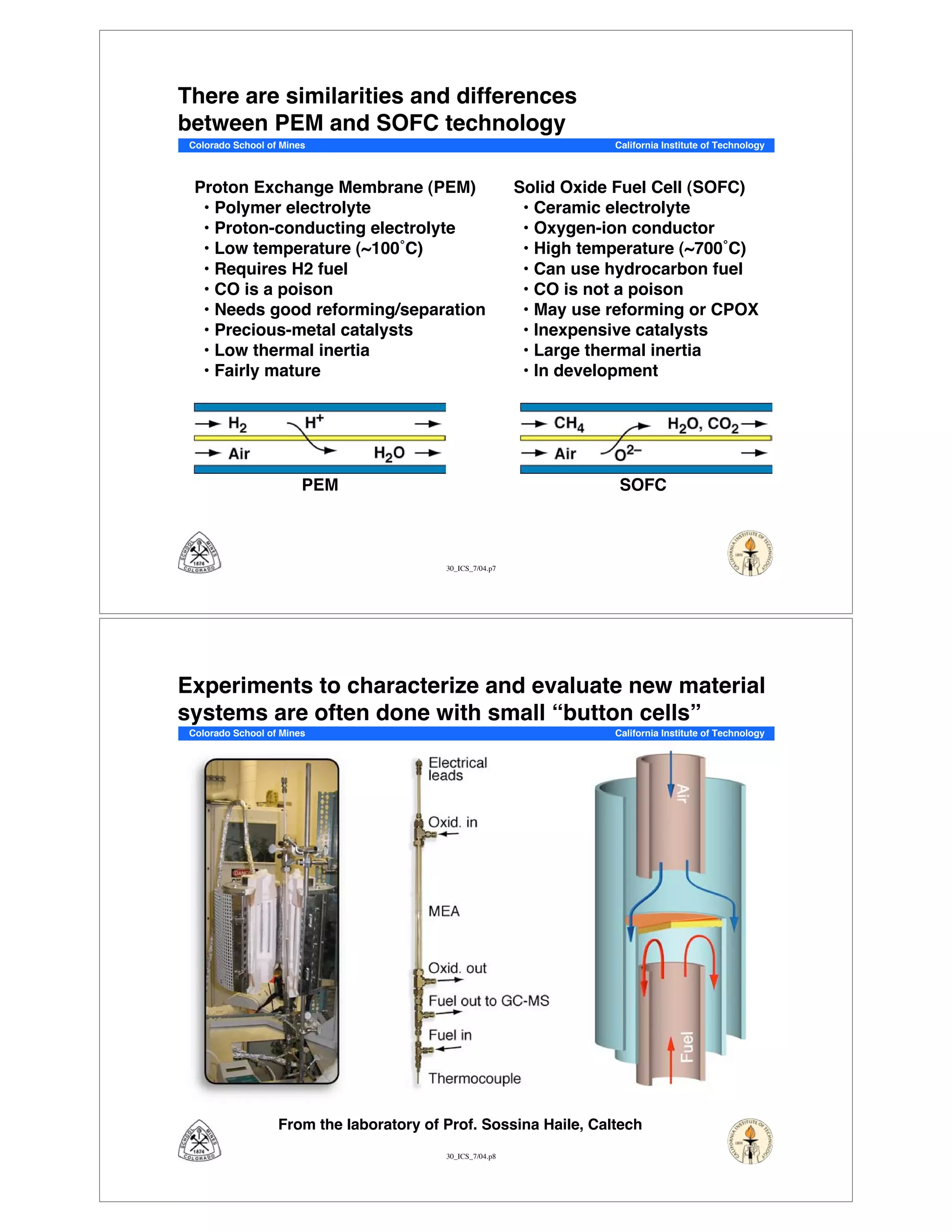
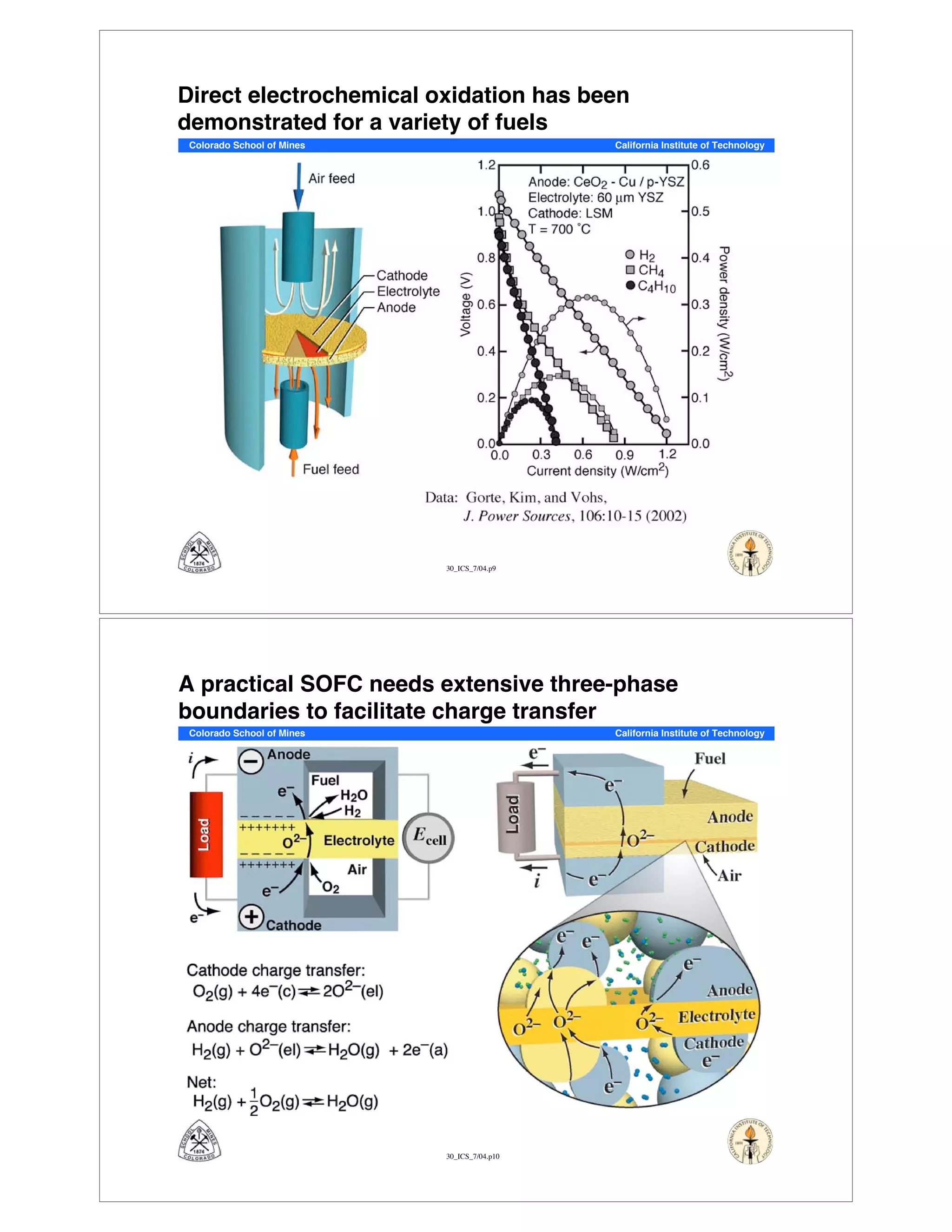
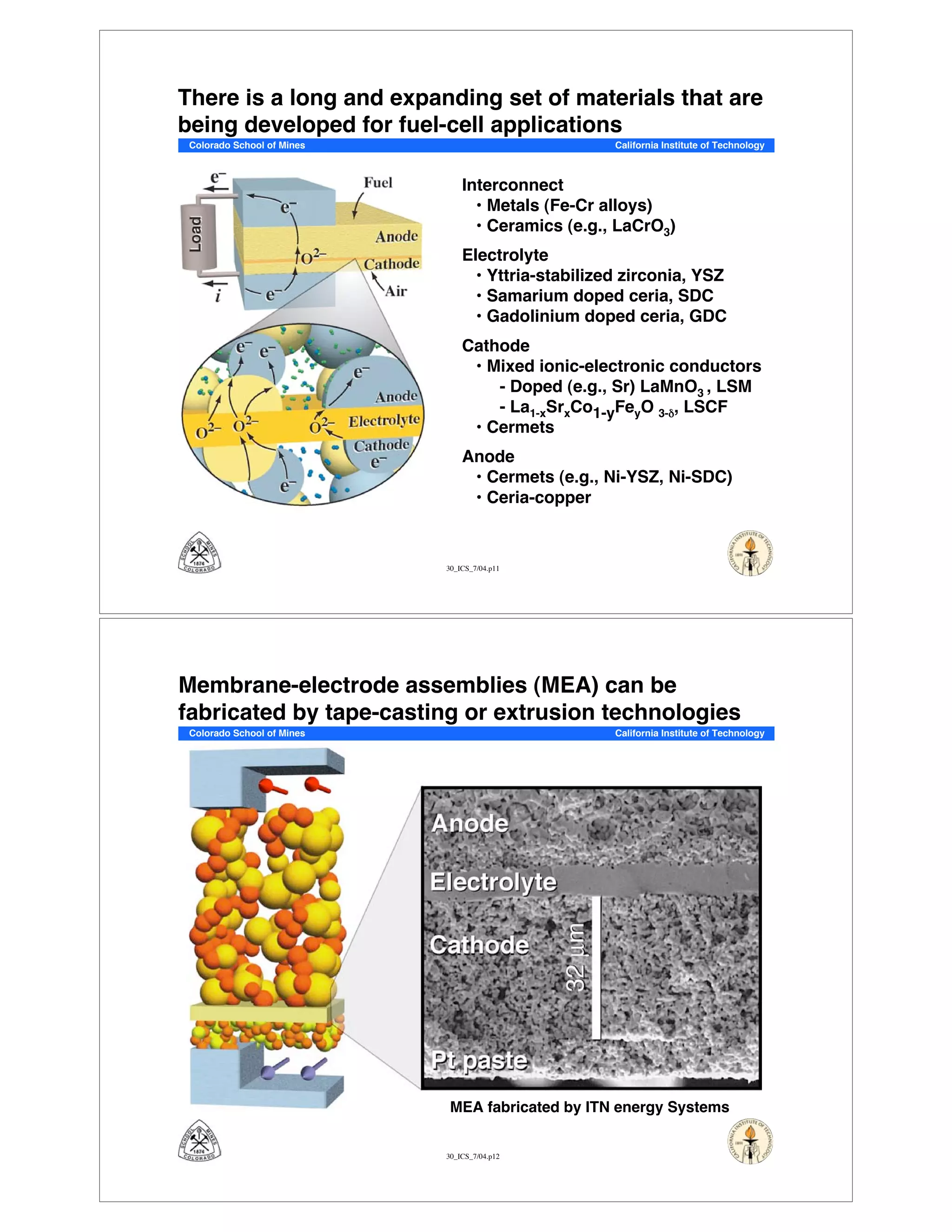
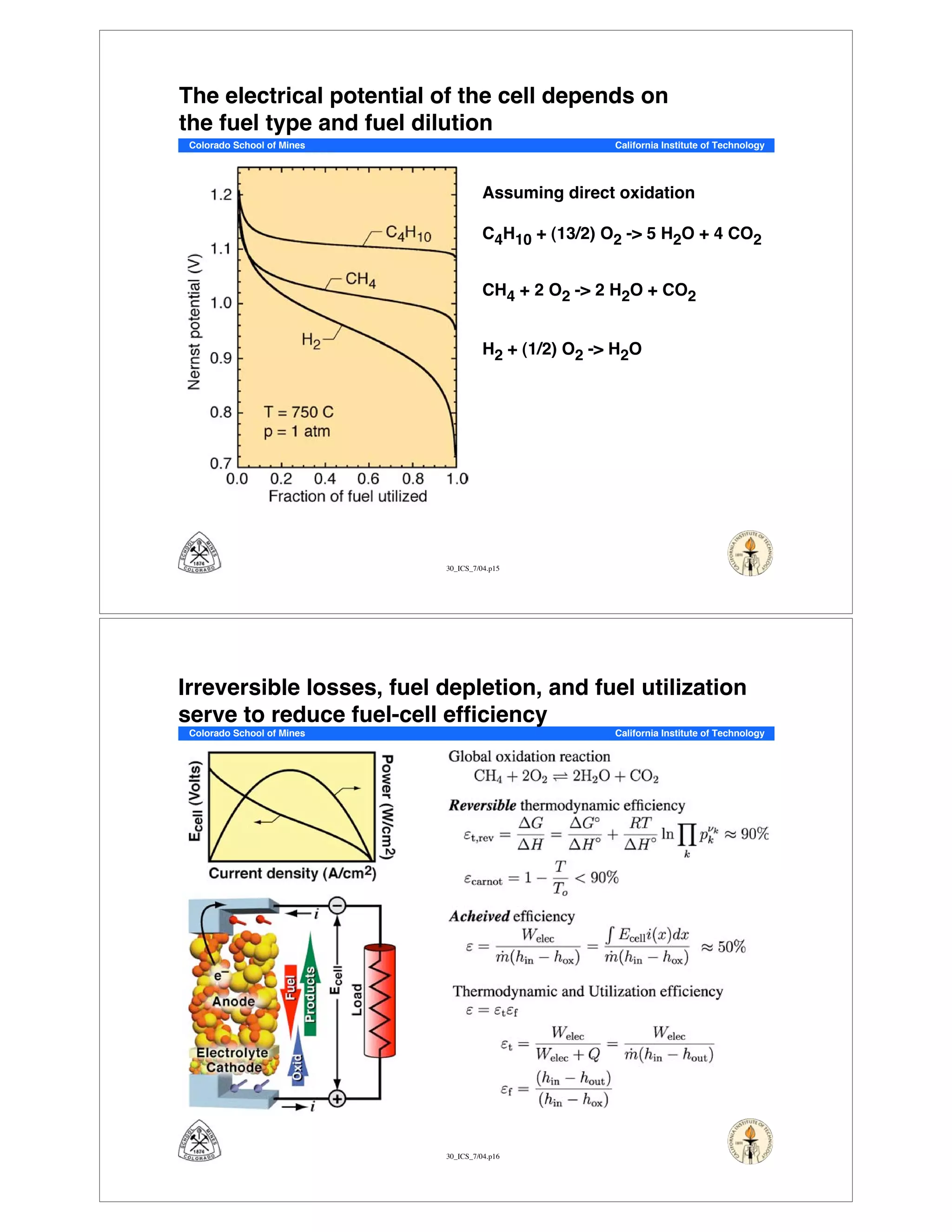
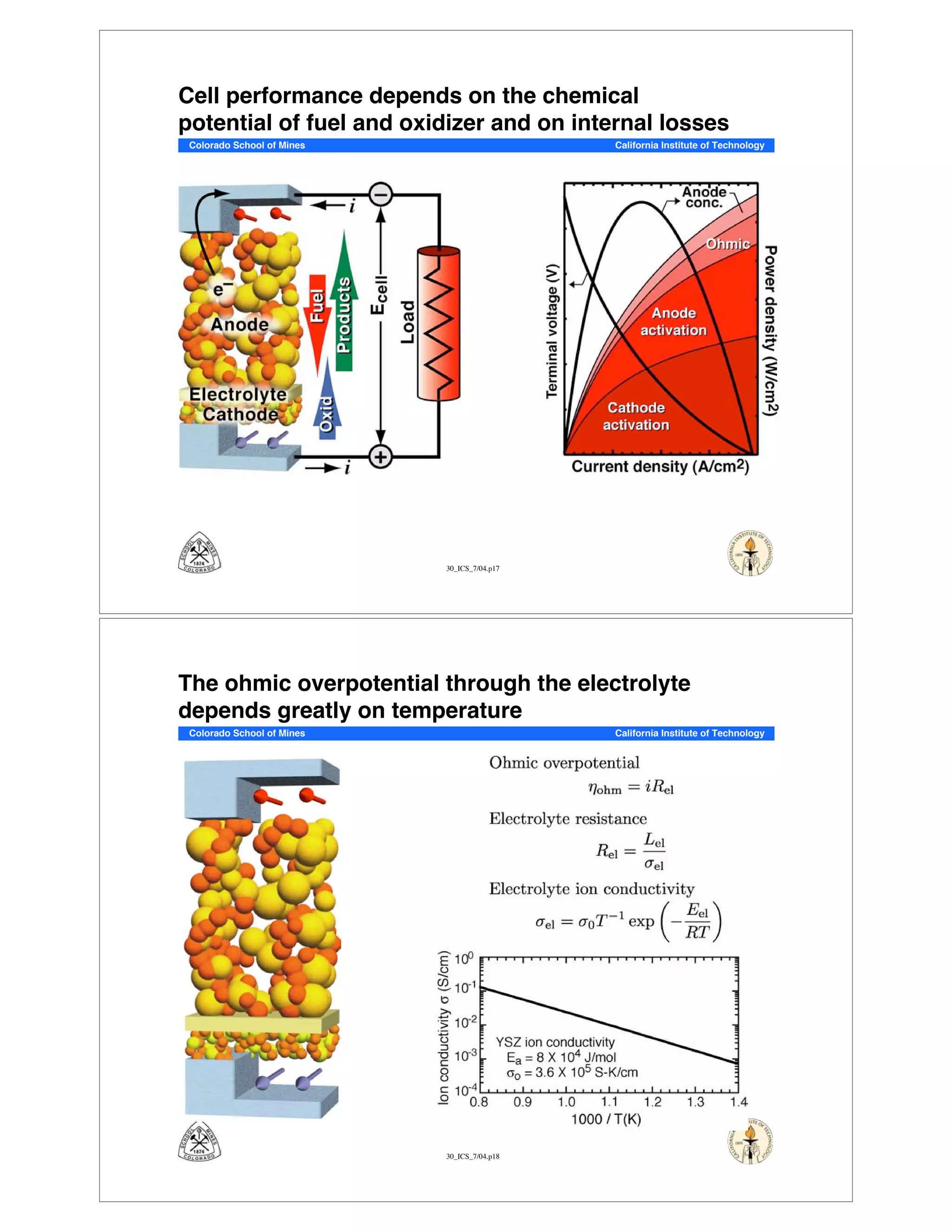
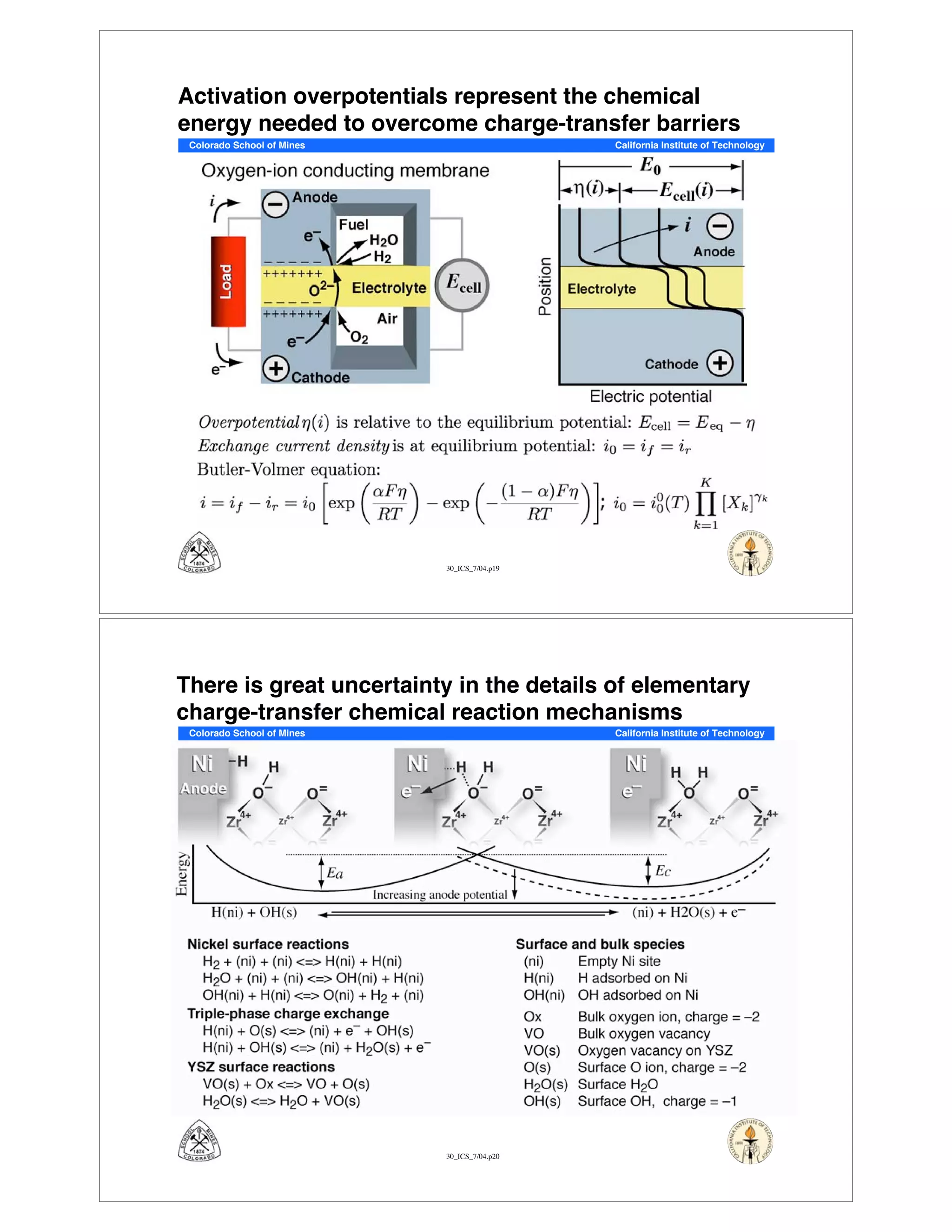
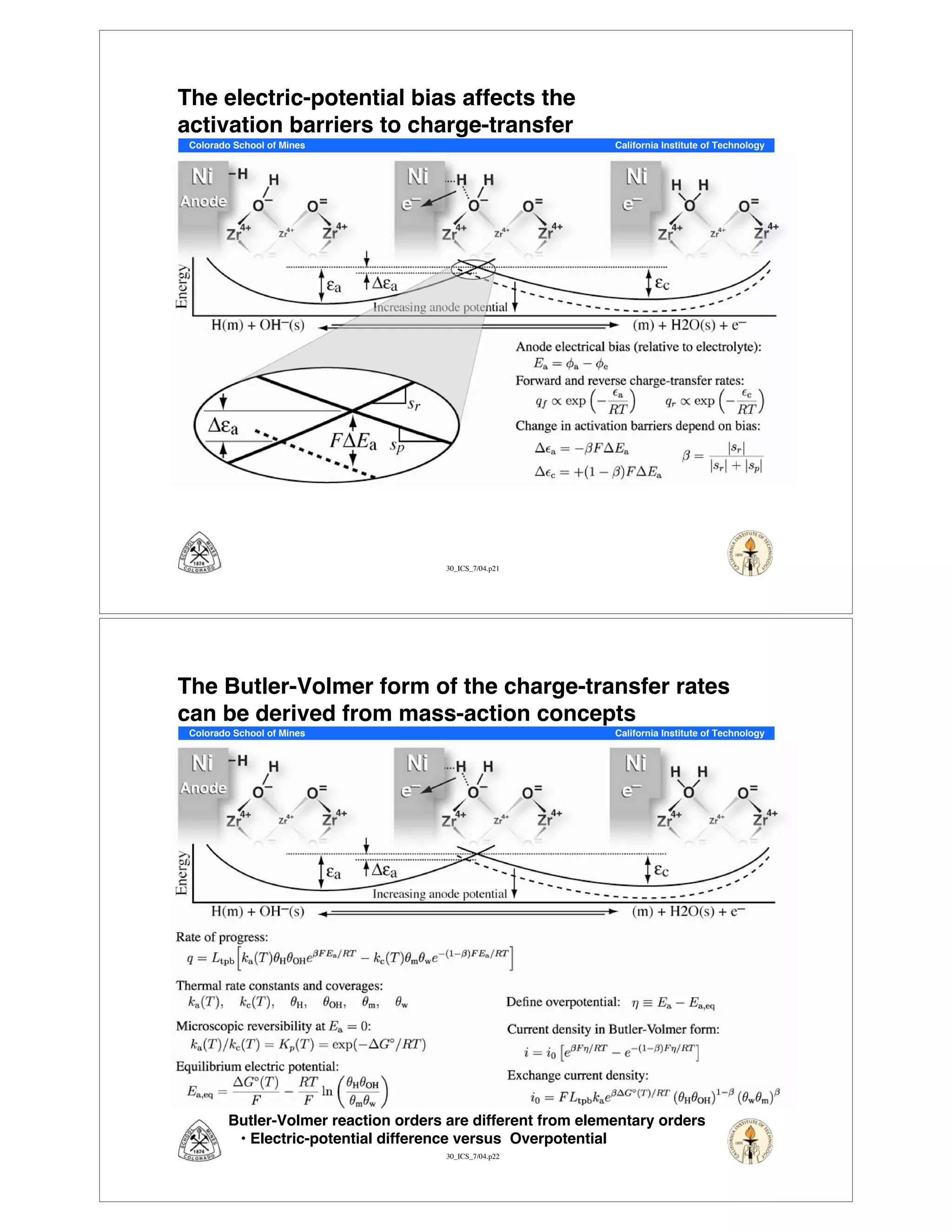
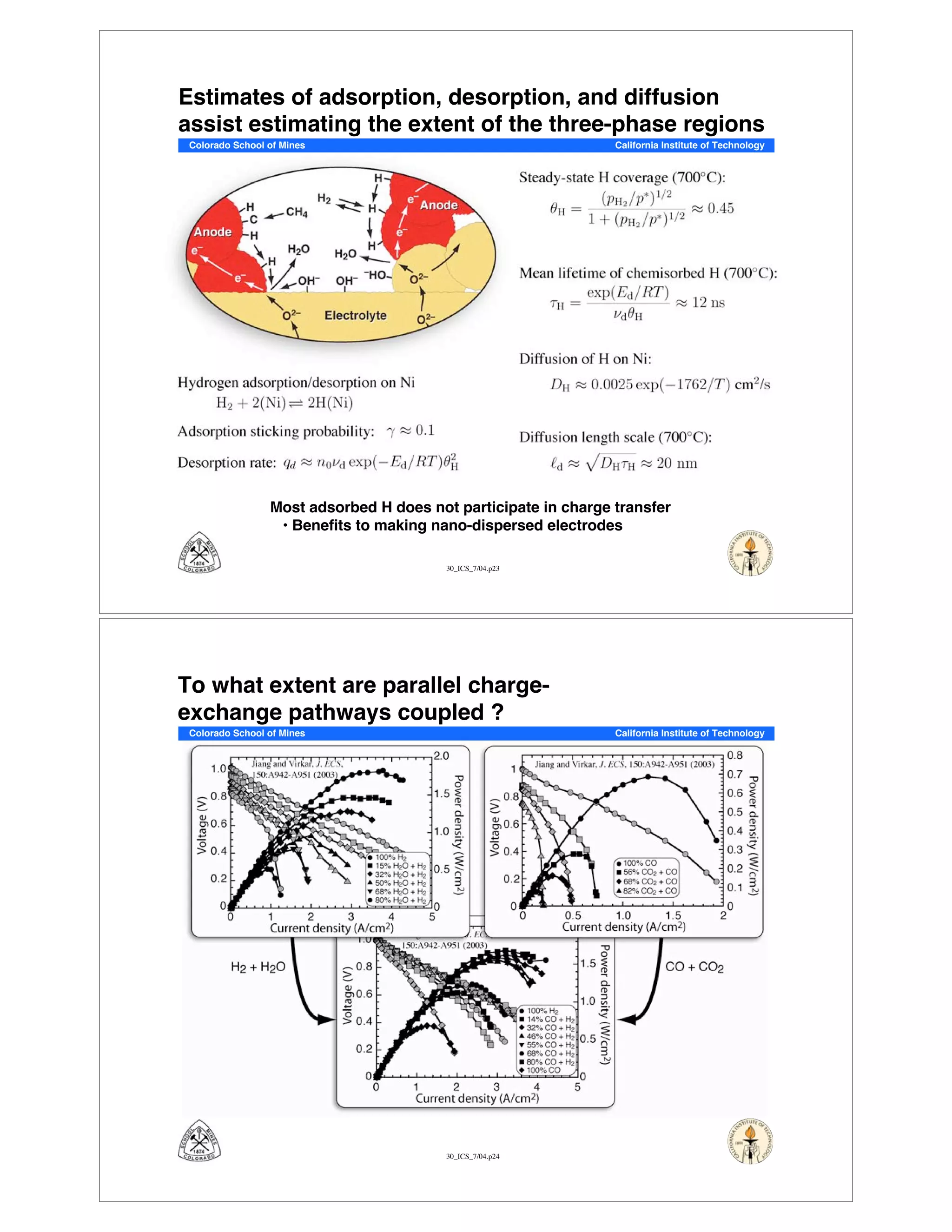
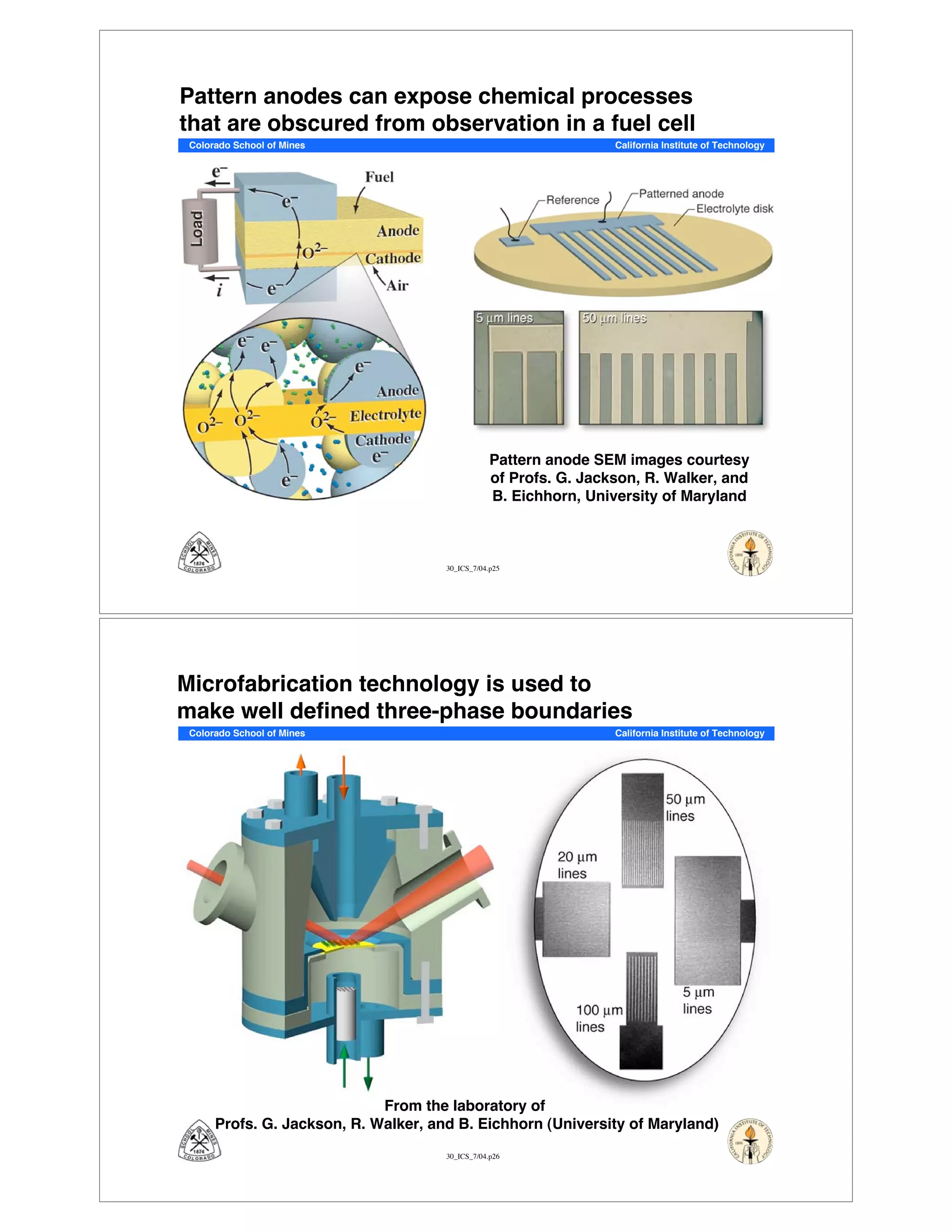
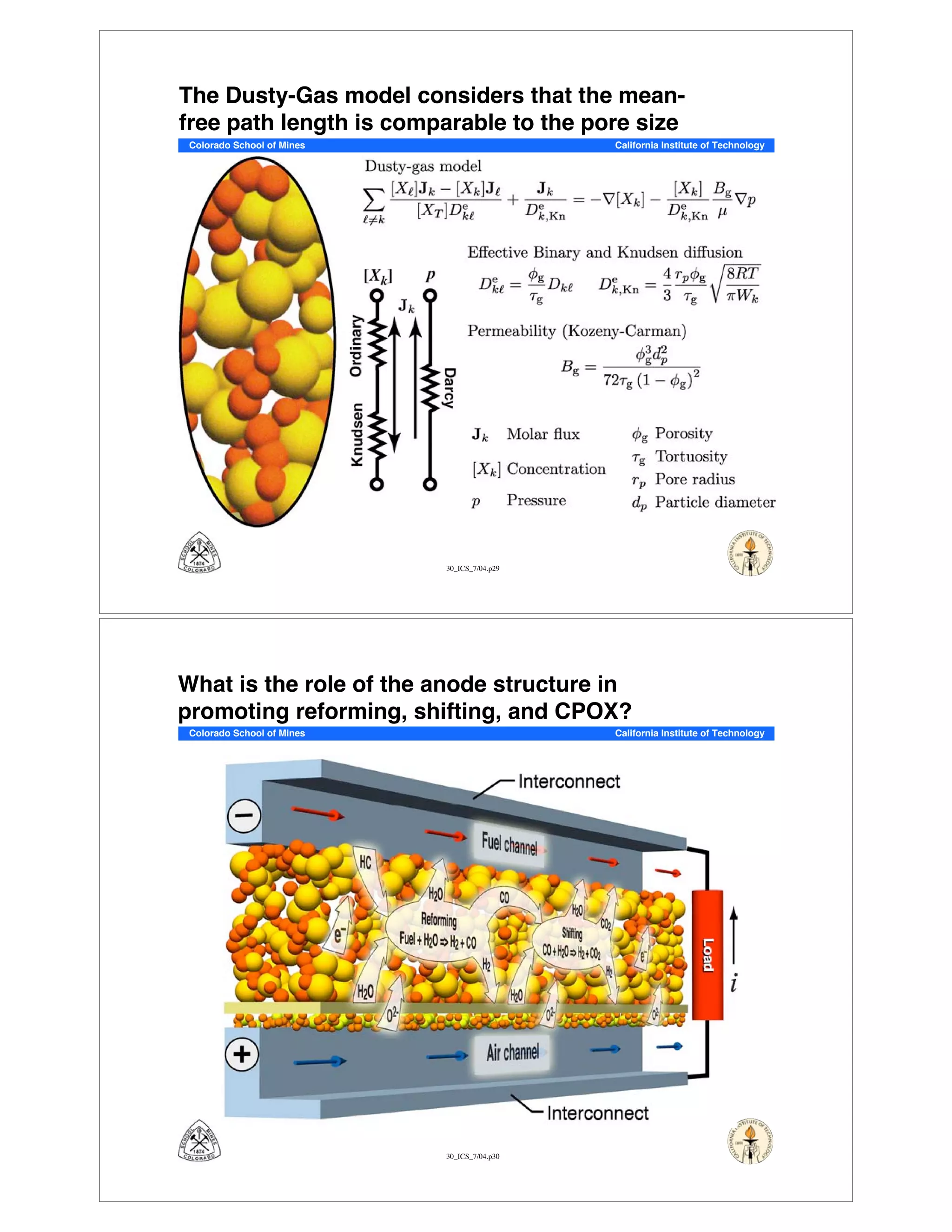
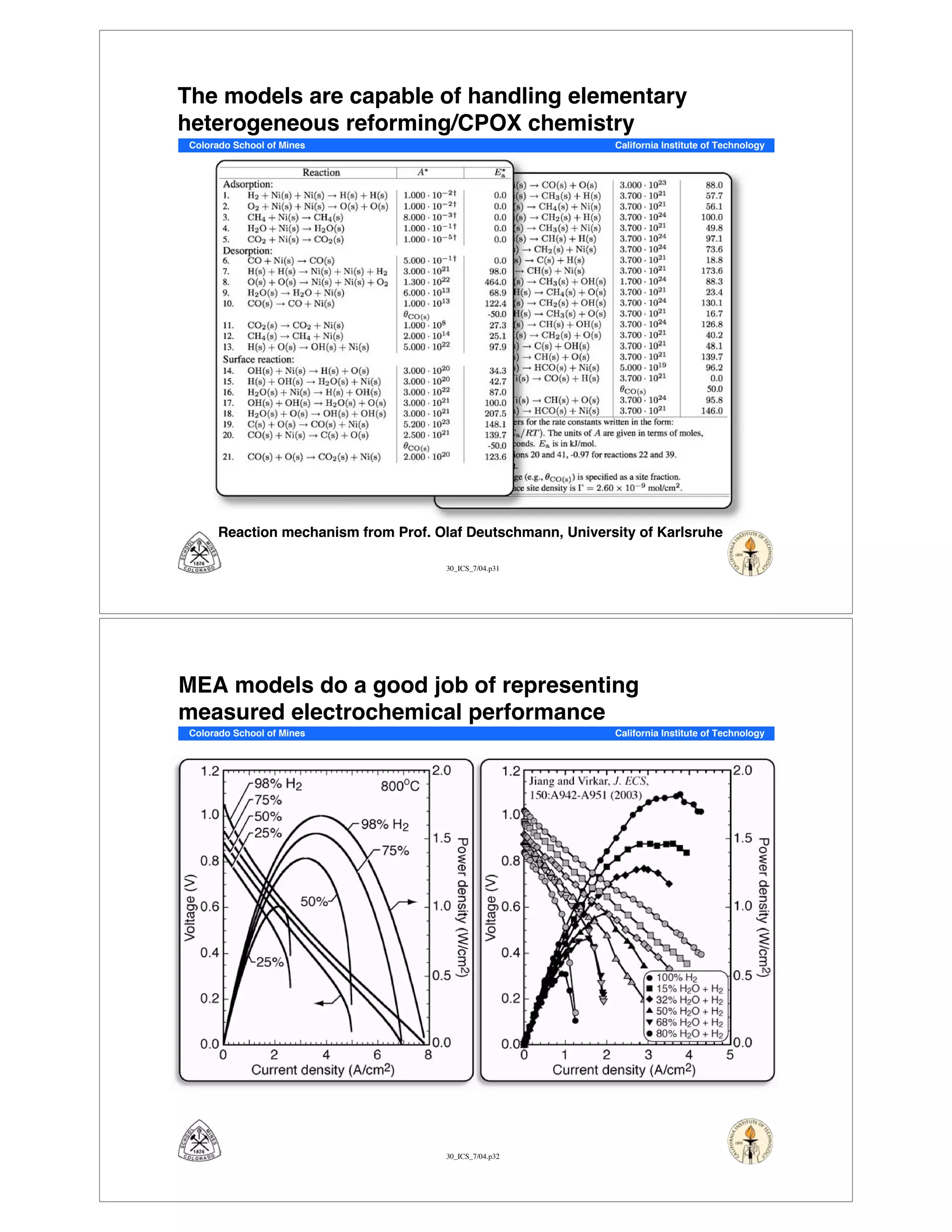
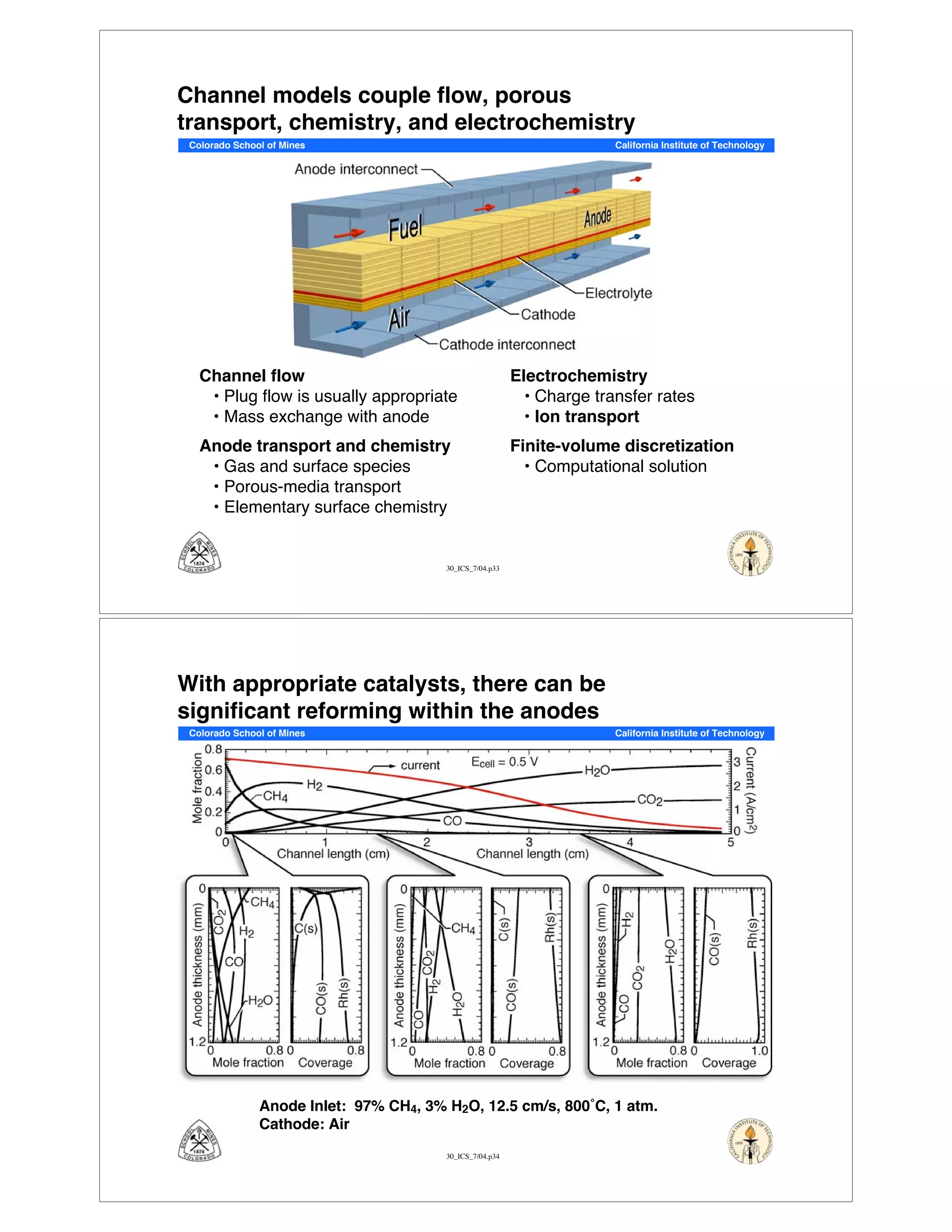
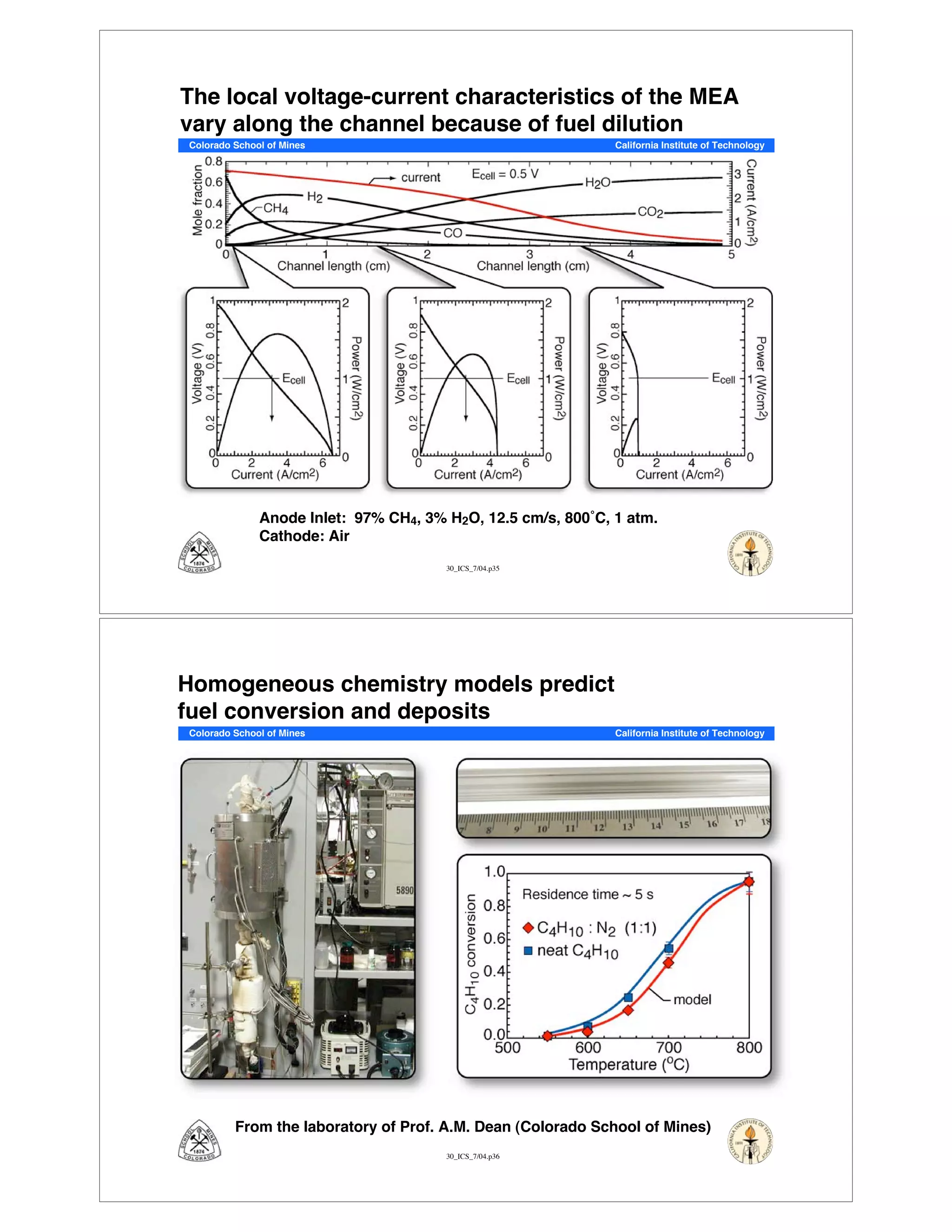
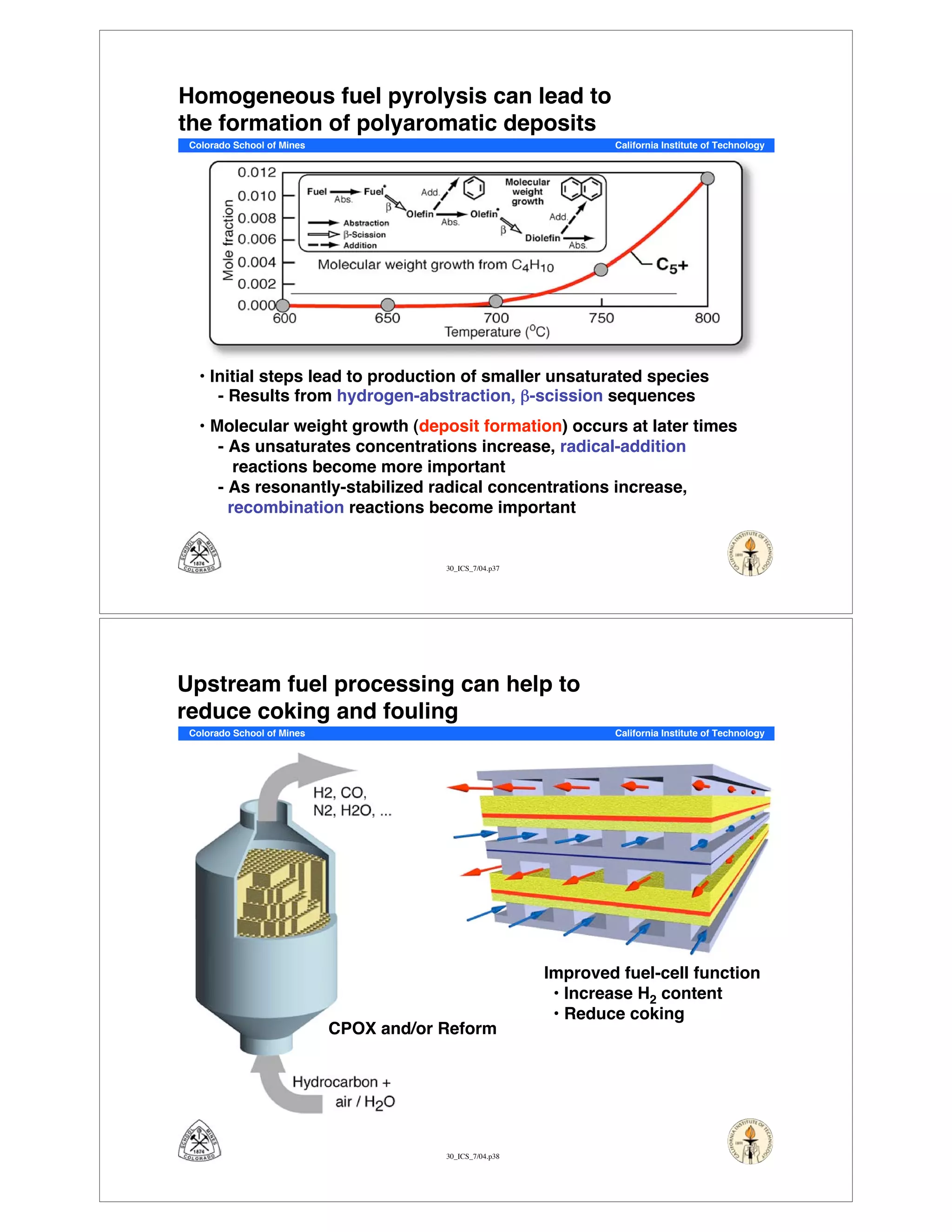
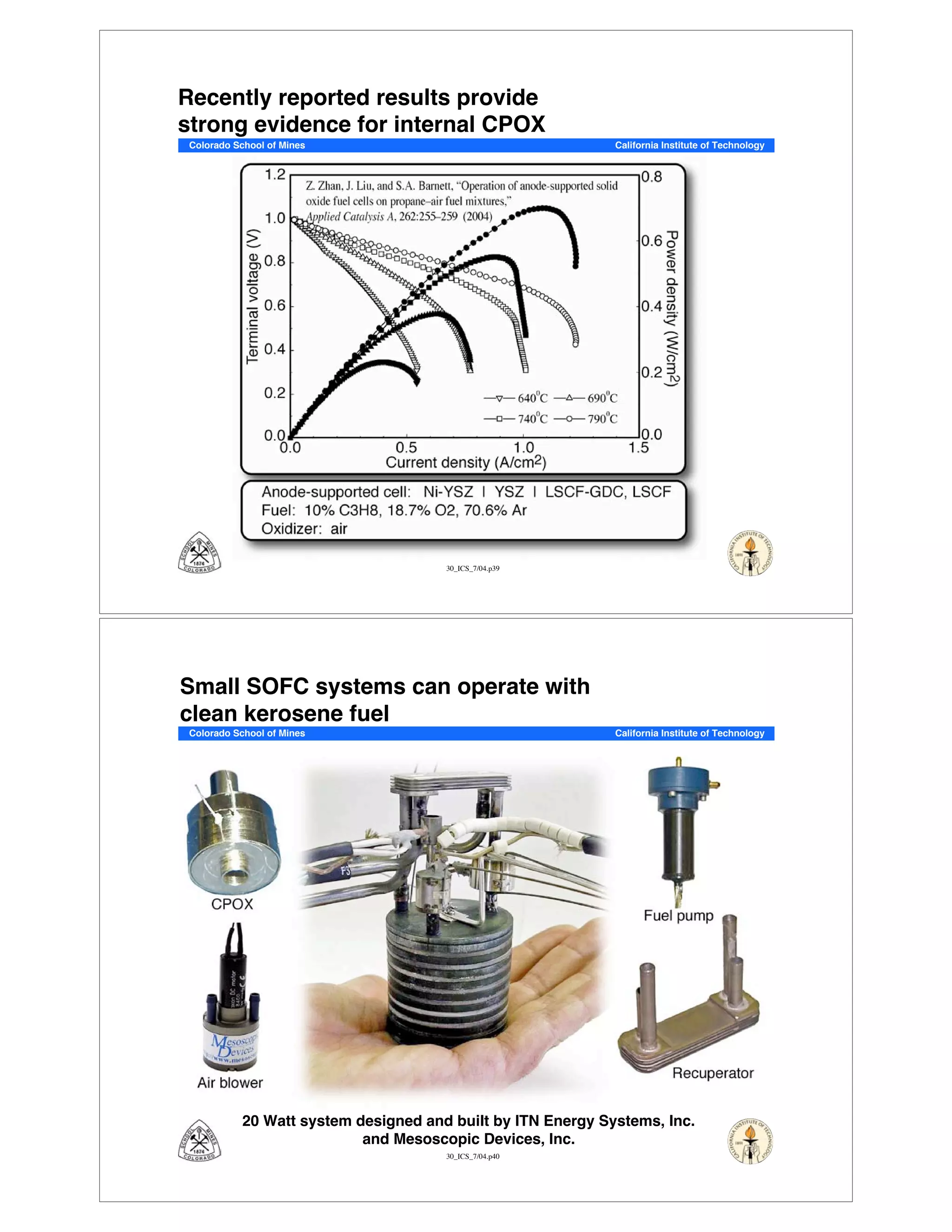
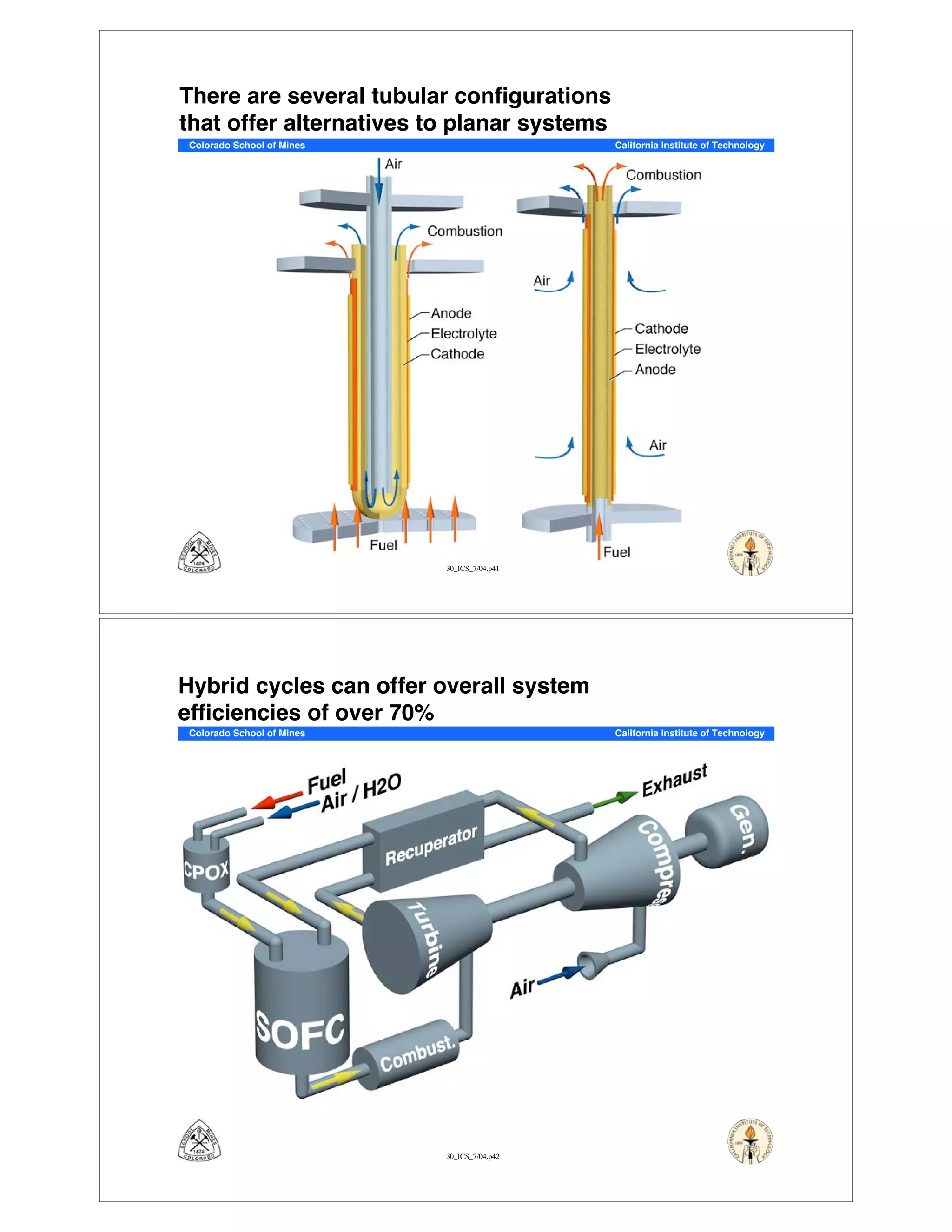
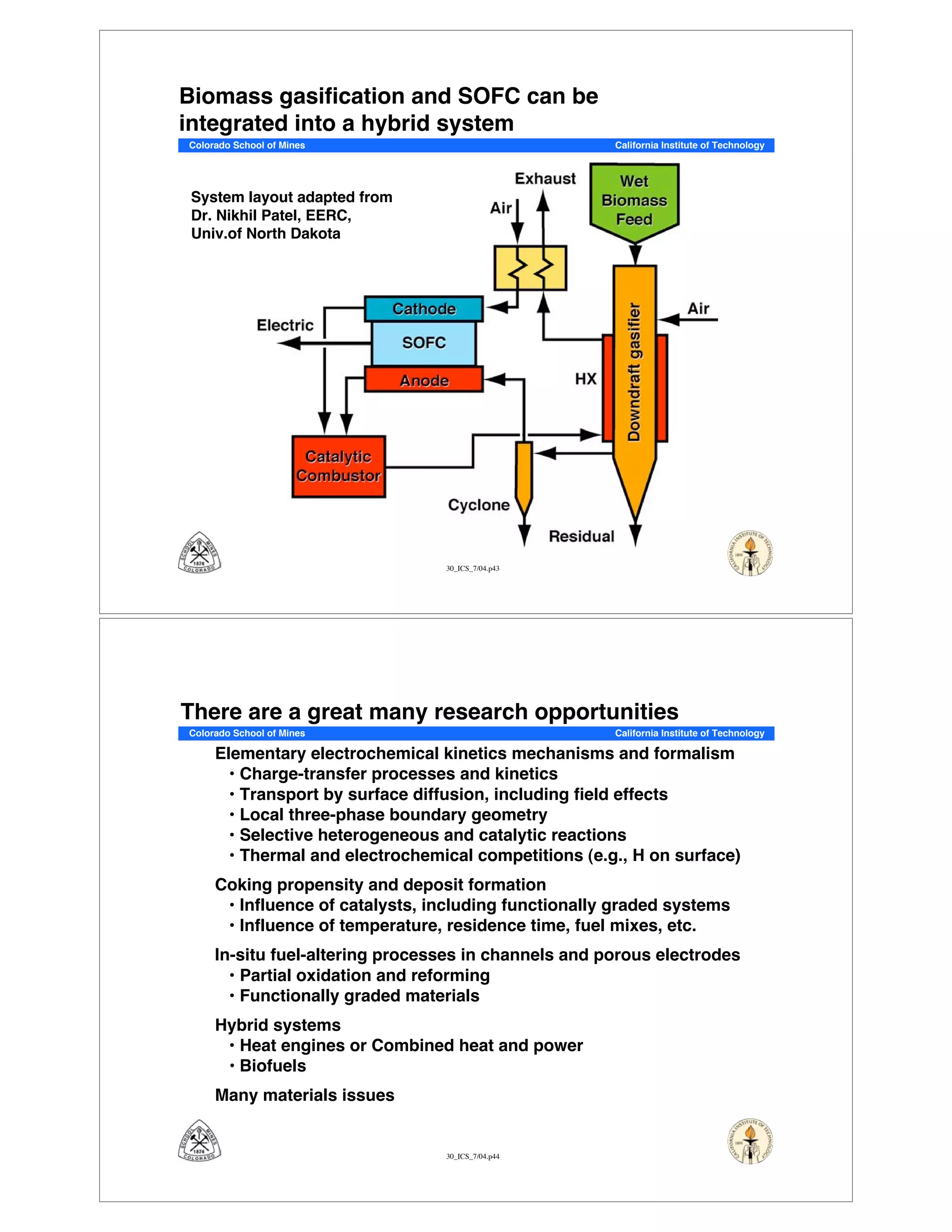
This document summarizes research on solid-oxide fuel cells (SOFCs) that can directly oxidize hydrocarbons and hydrocarbon fuels. It discusses several benefits of SOFCs, including that they can operate at high temperatures and use hydrocarbon fuels without being poisoned by carbon monoxide. The document outlines SOFC components and operating principles. It also summarizes research on materials, cell performance, chemical reaction mechanisms, modeling, and potential applications and research opportunities in SOFCs.