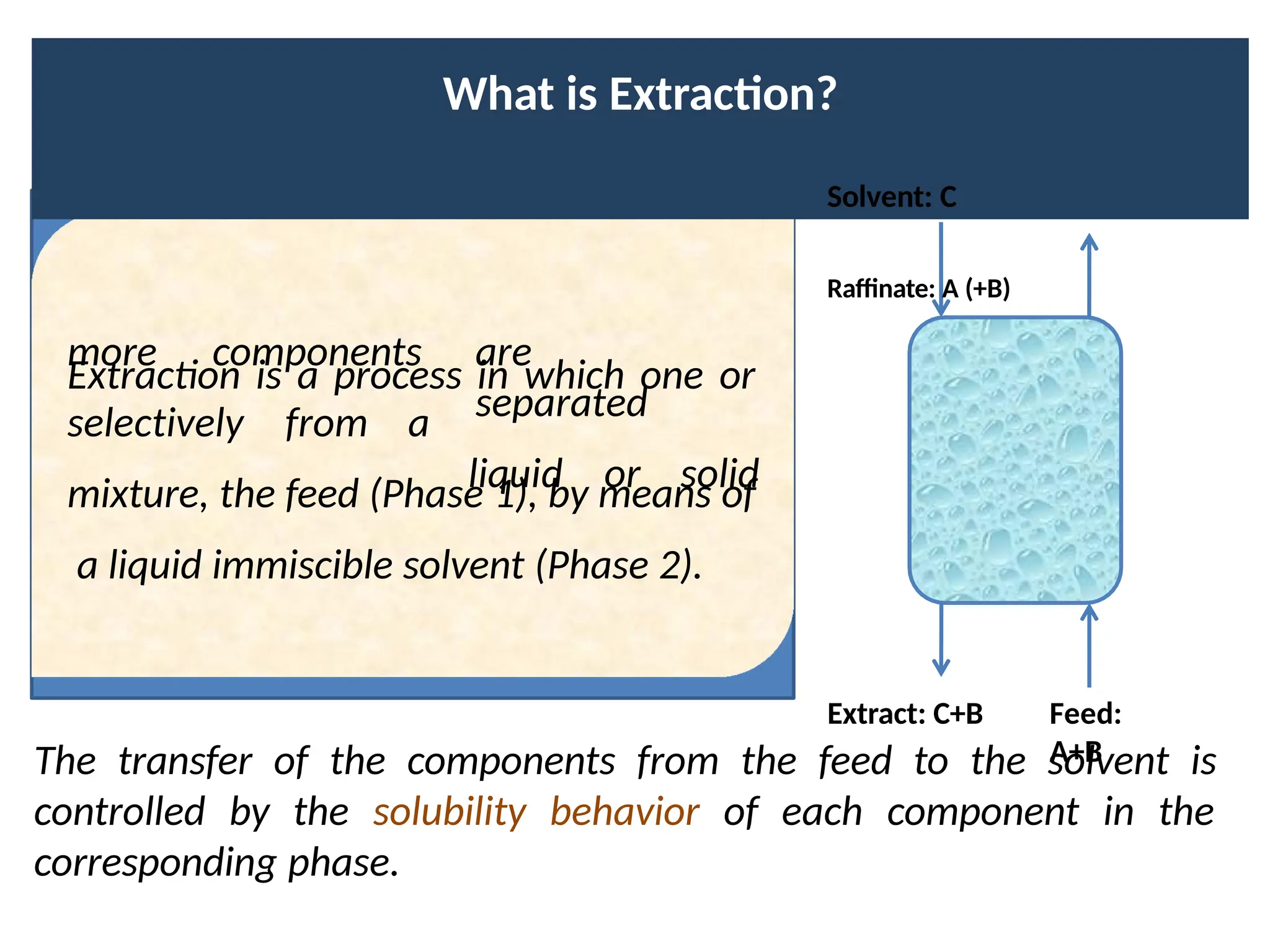
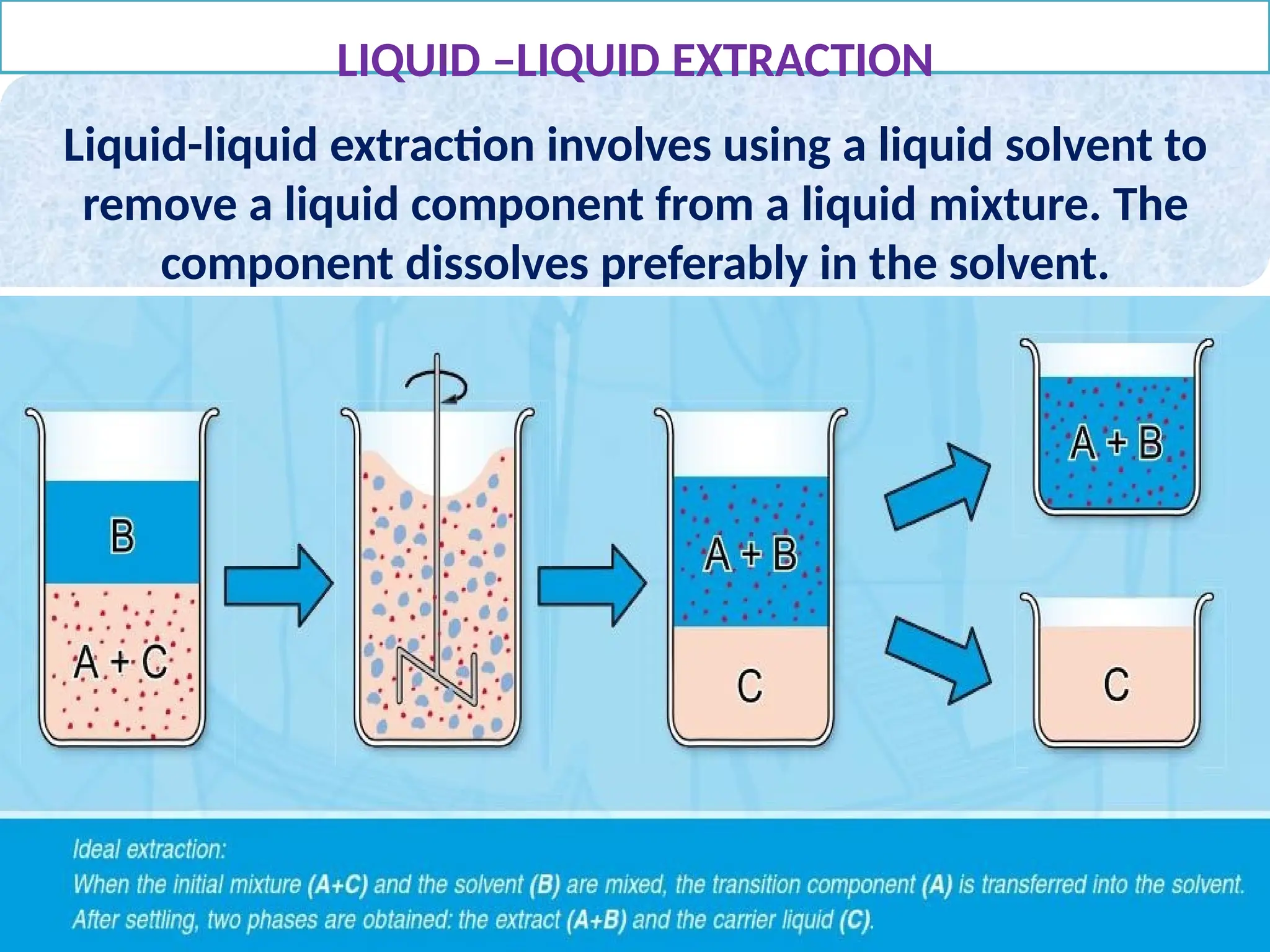
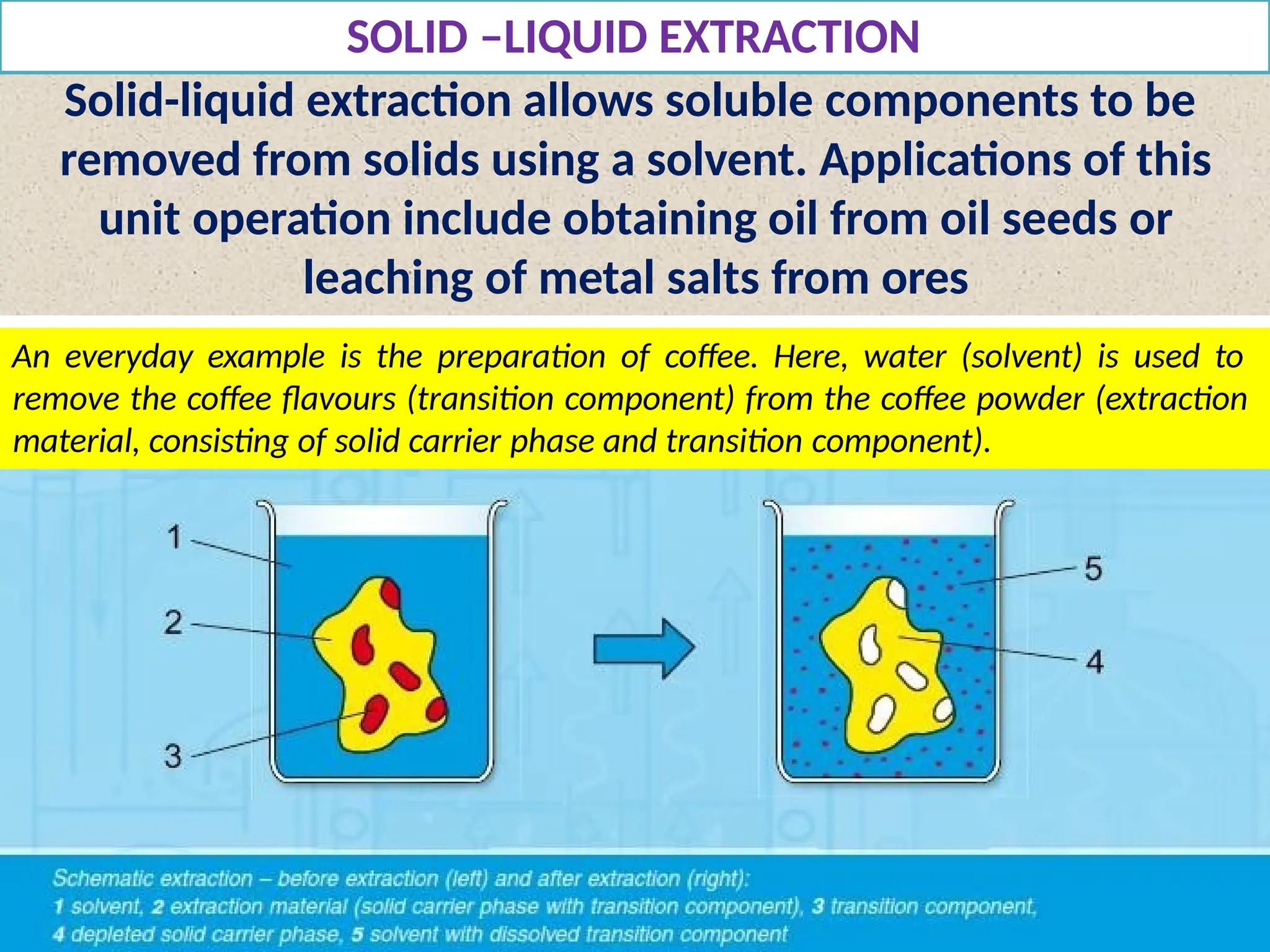
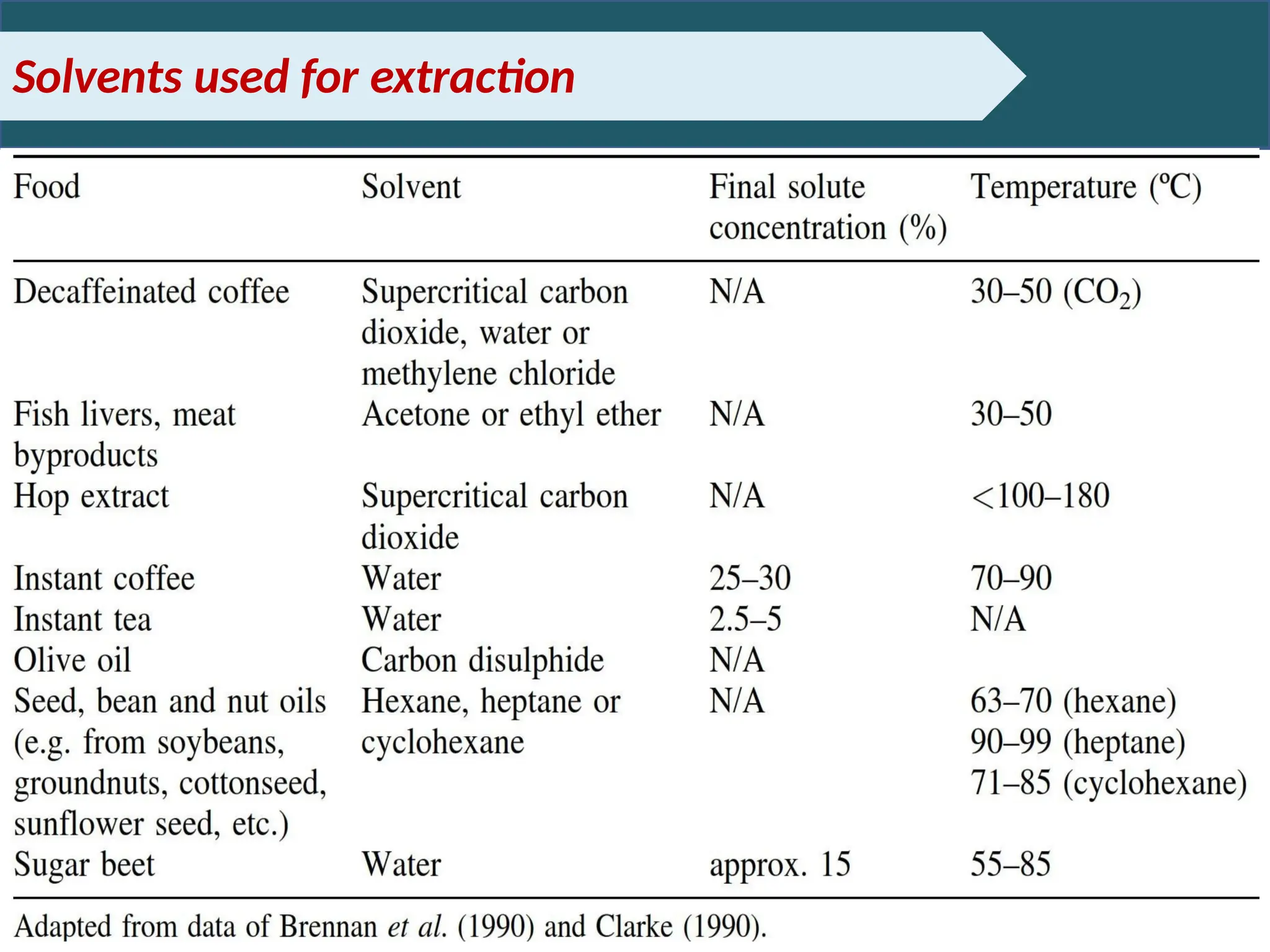
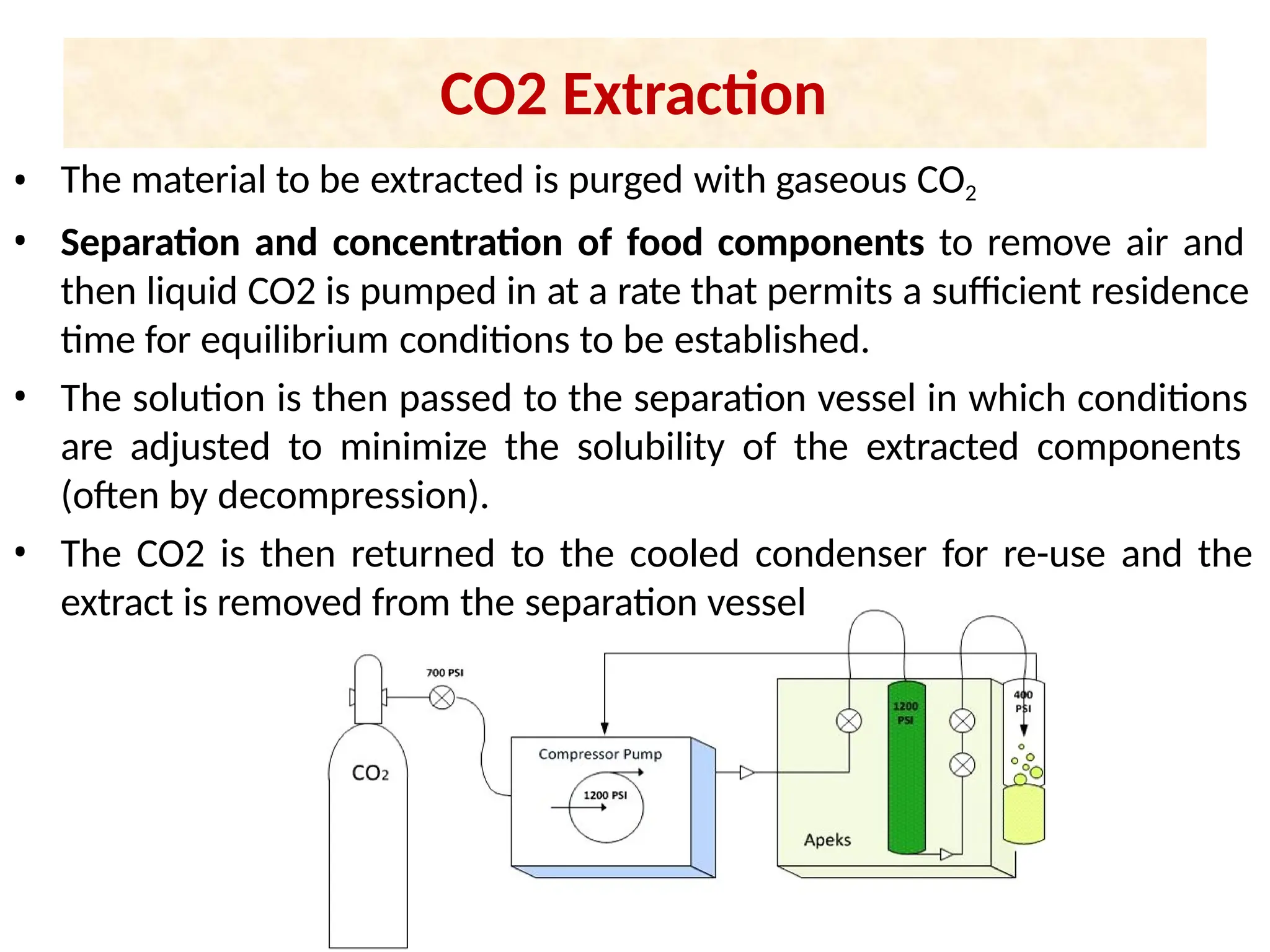
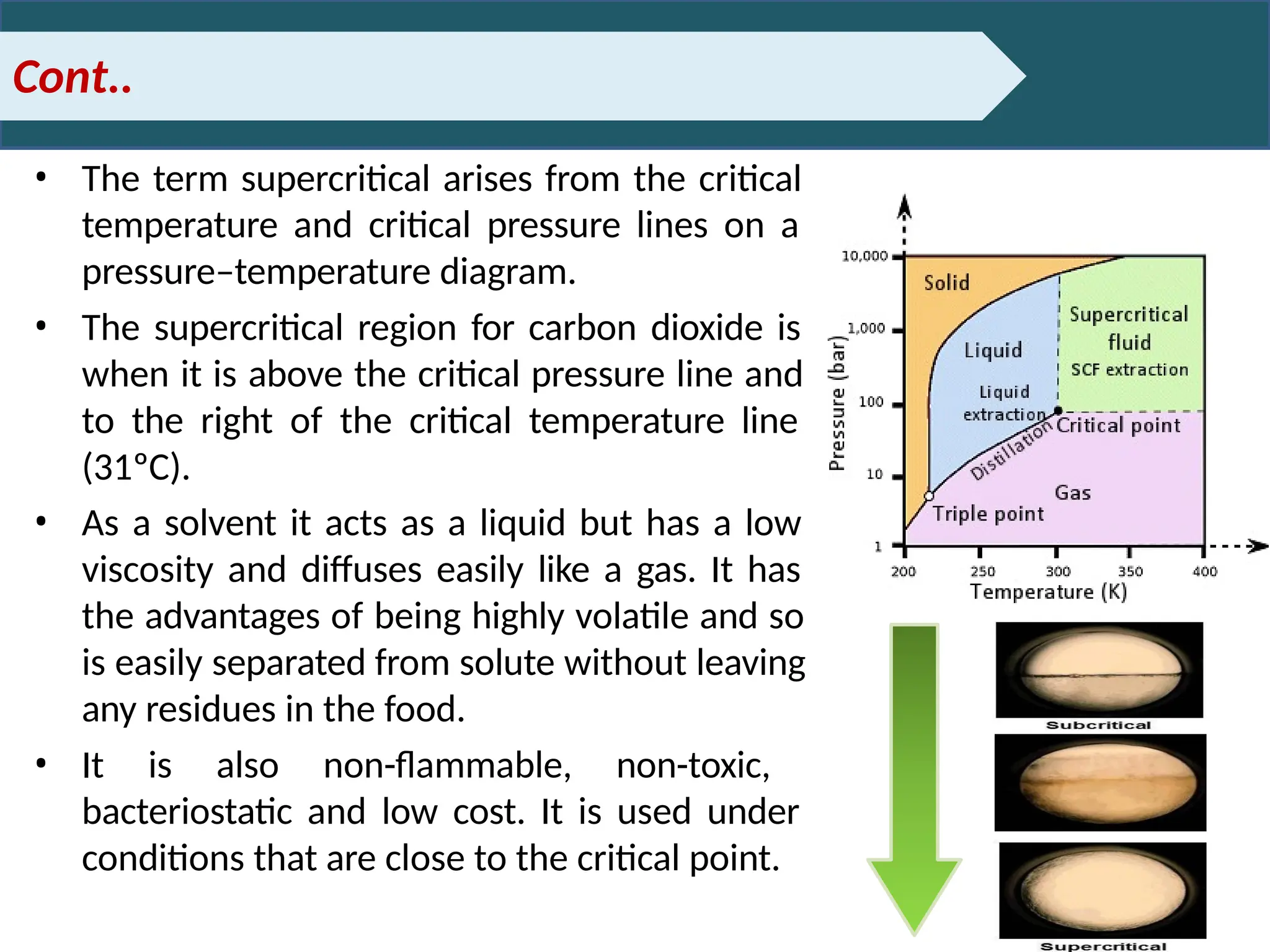
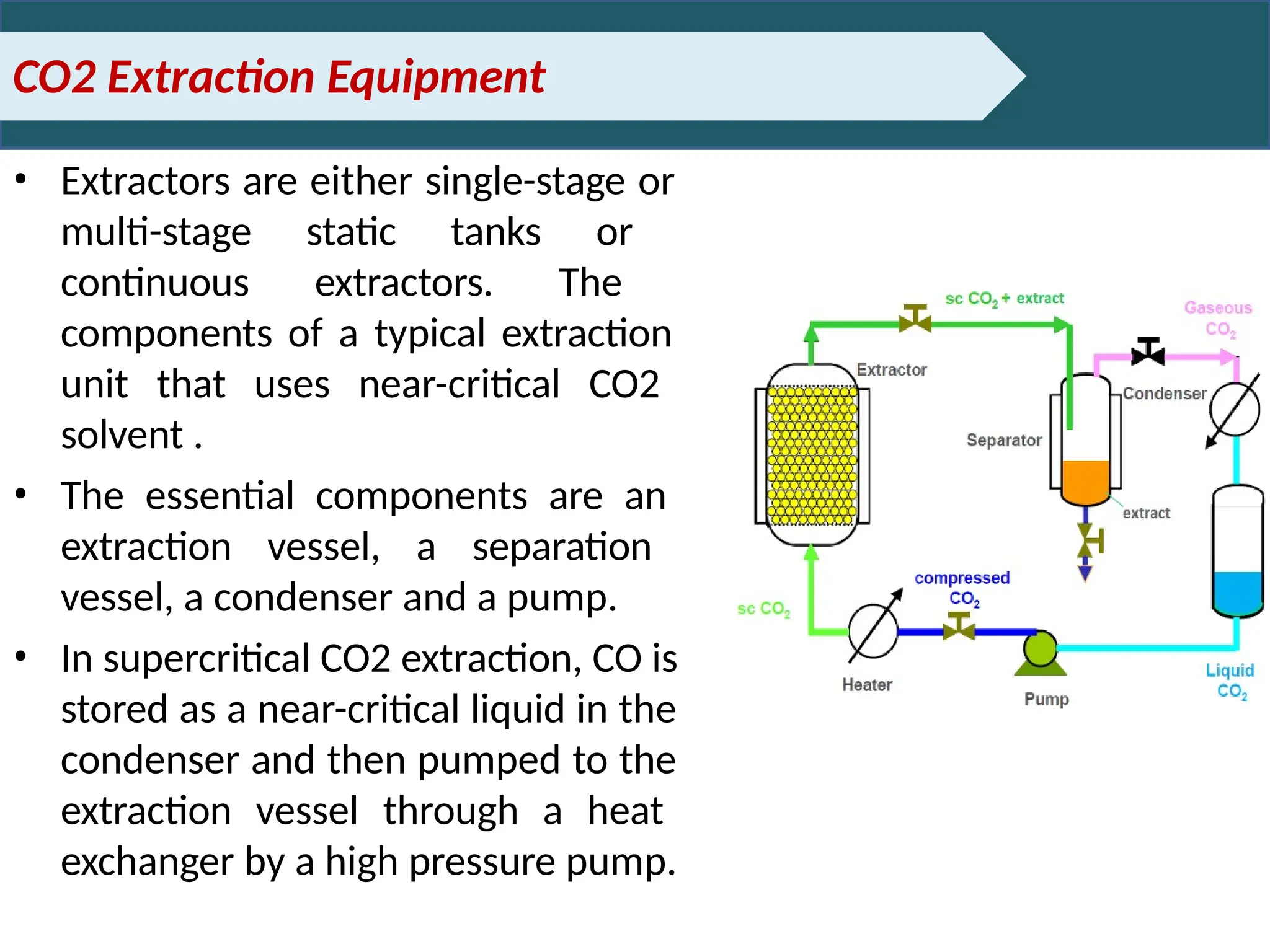
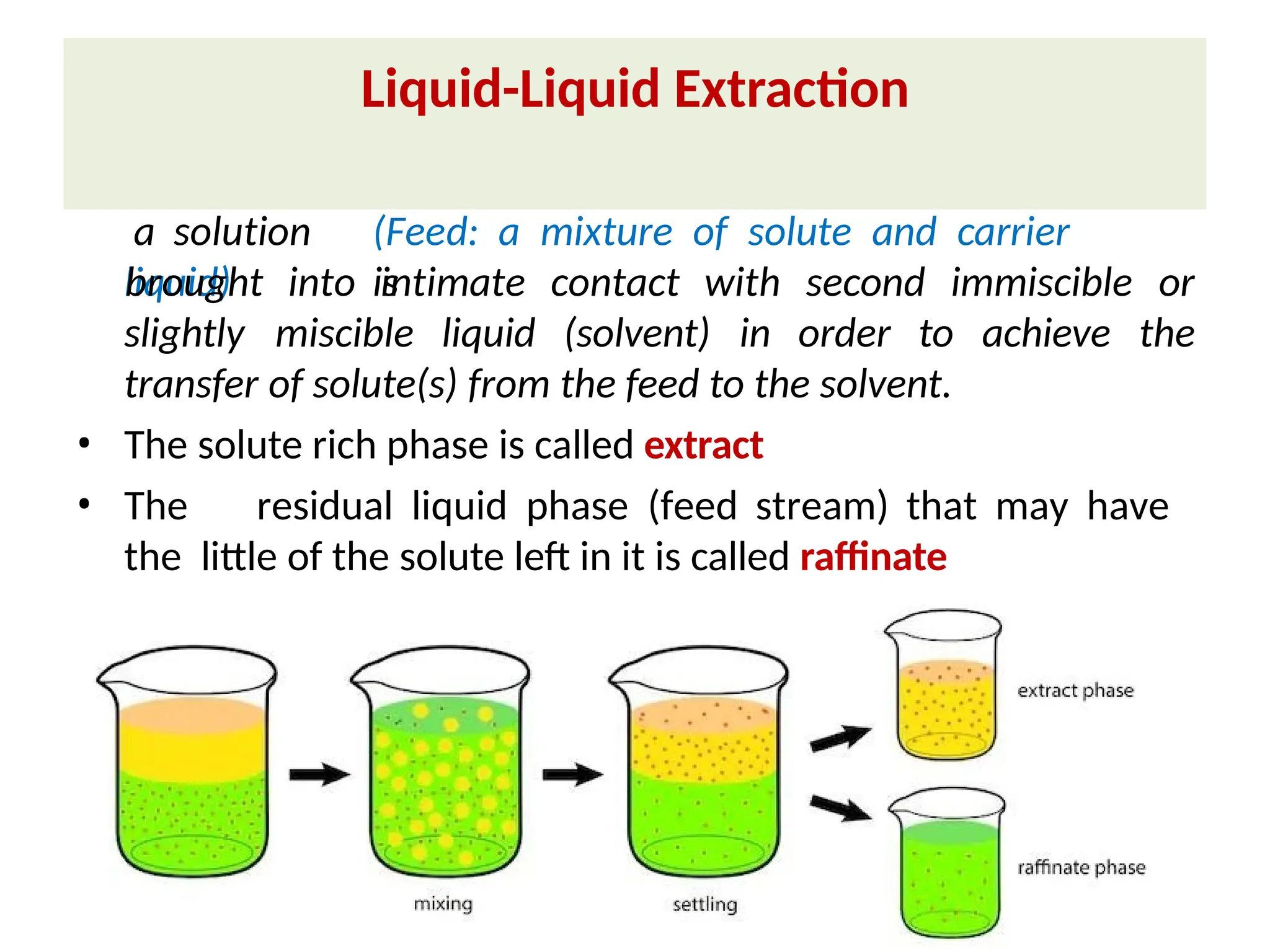
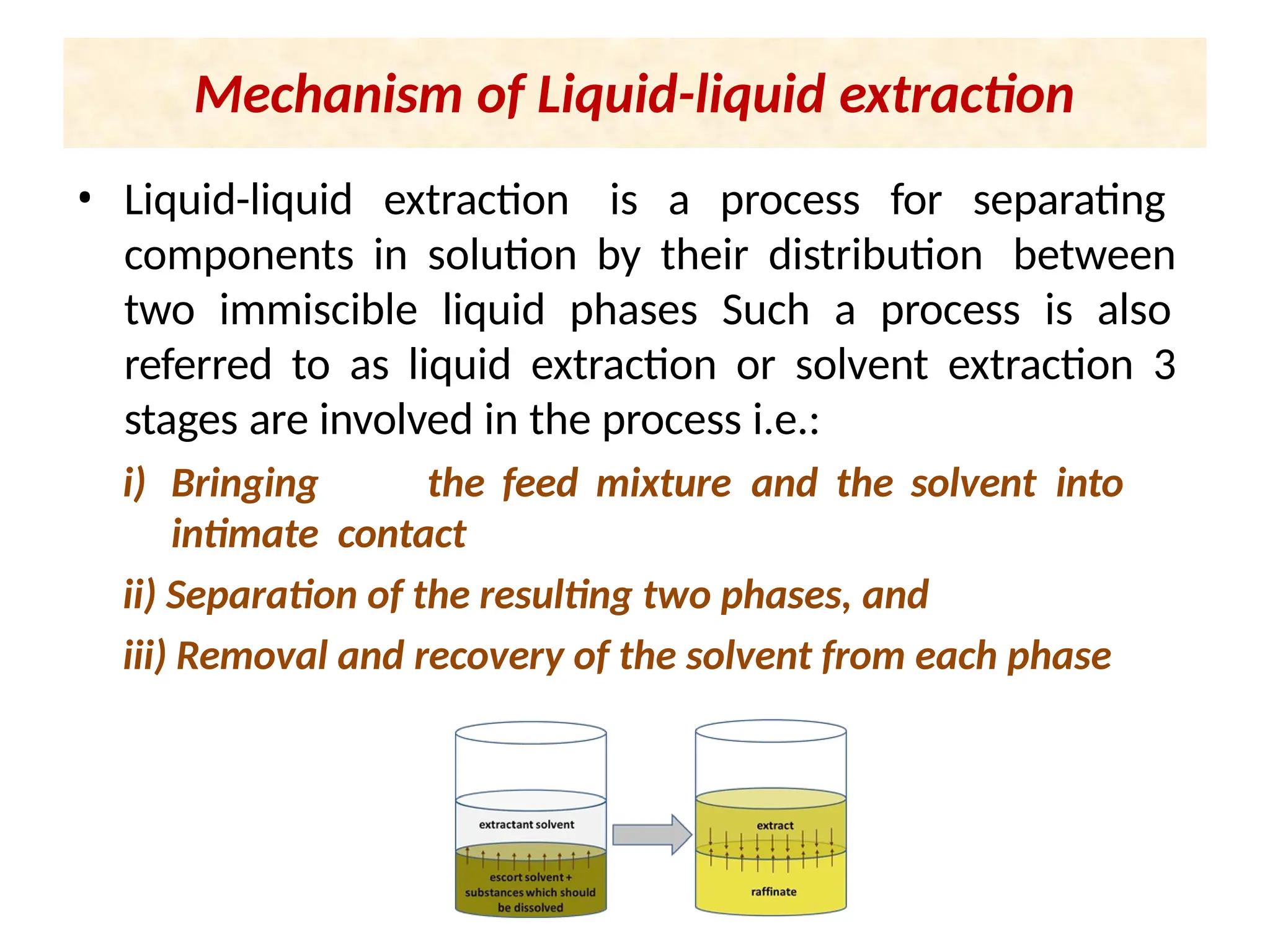
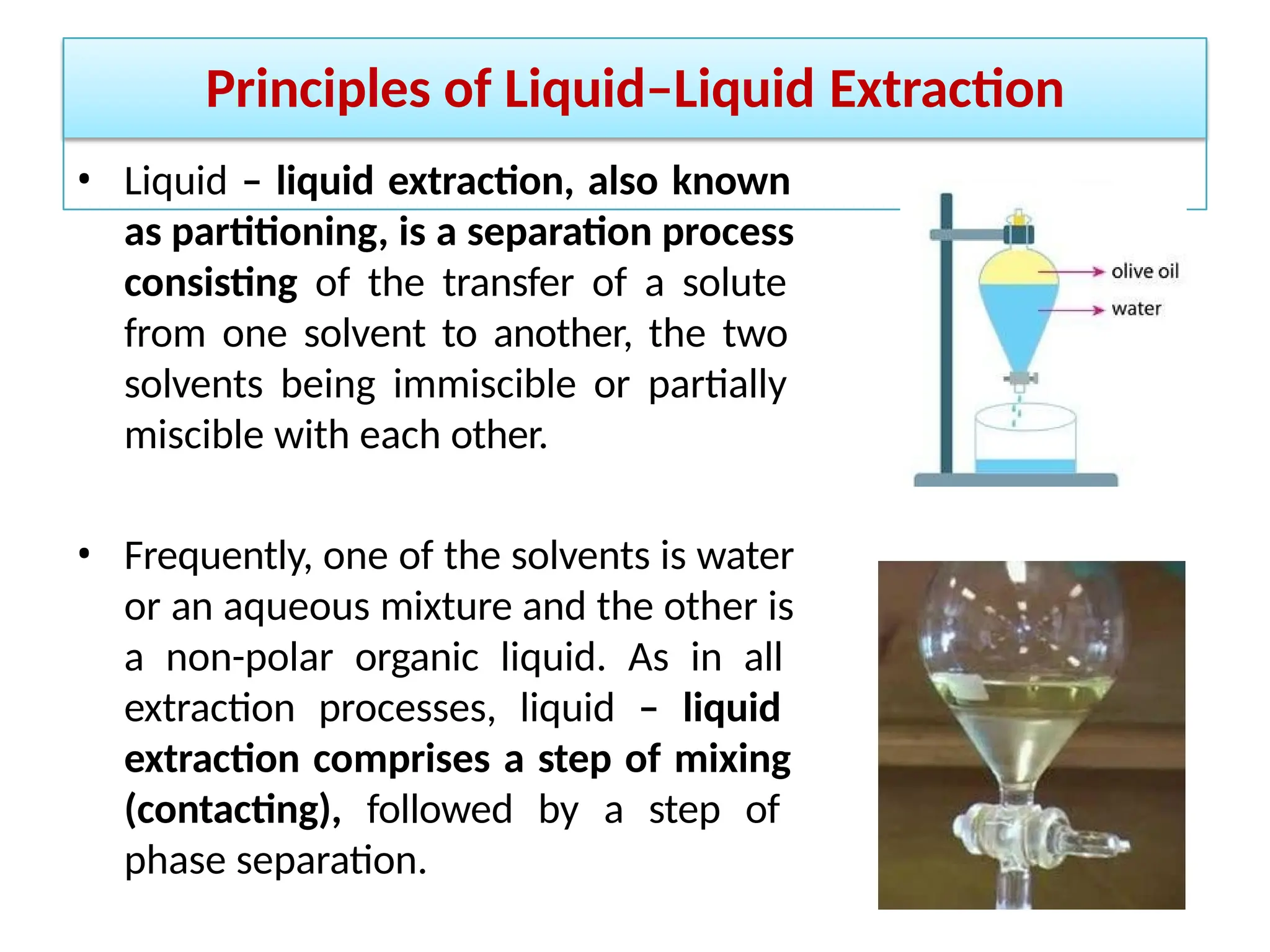
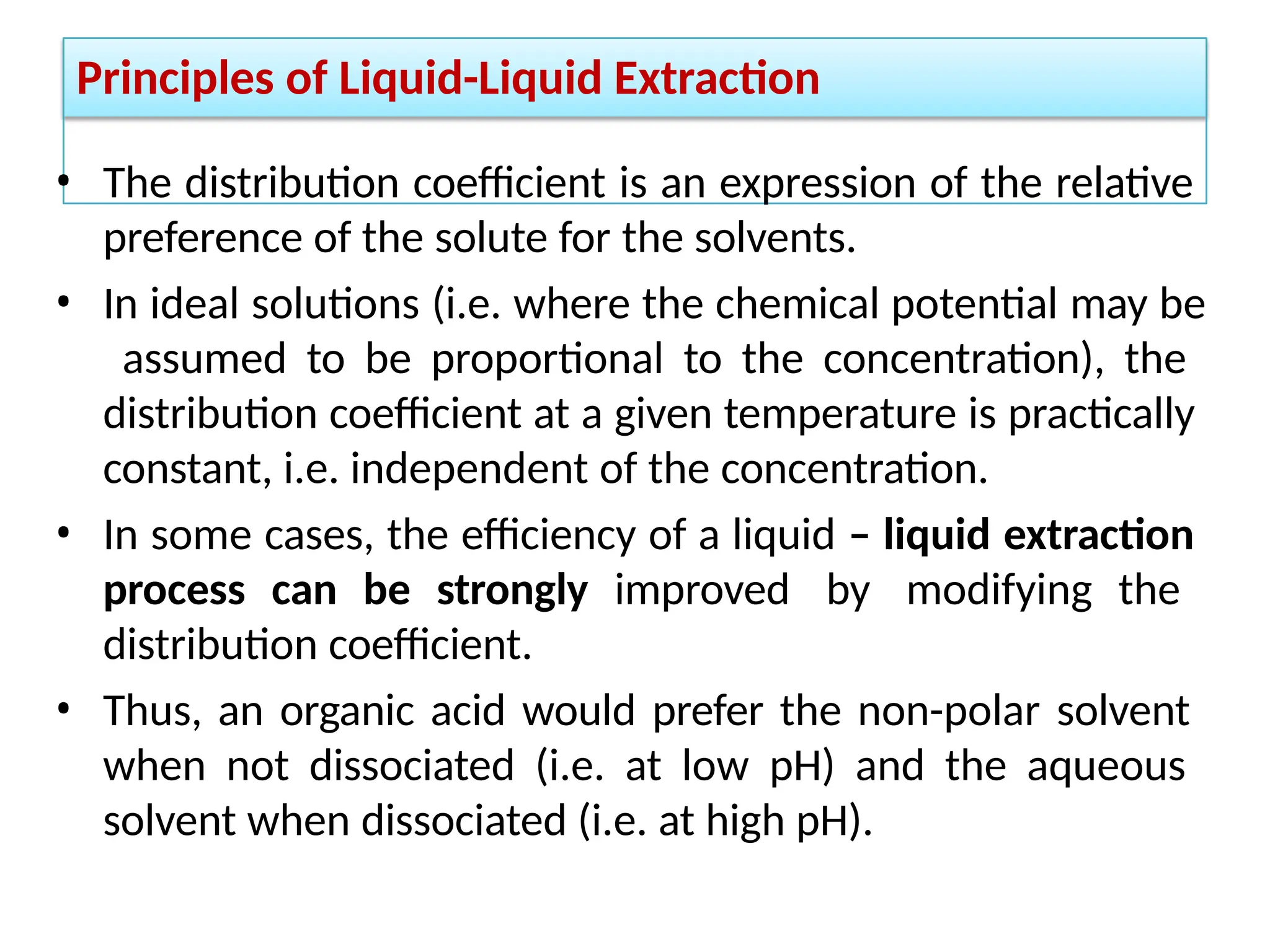
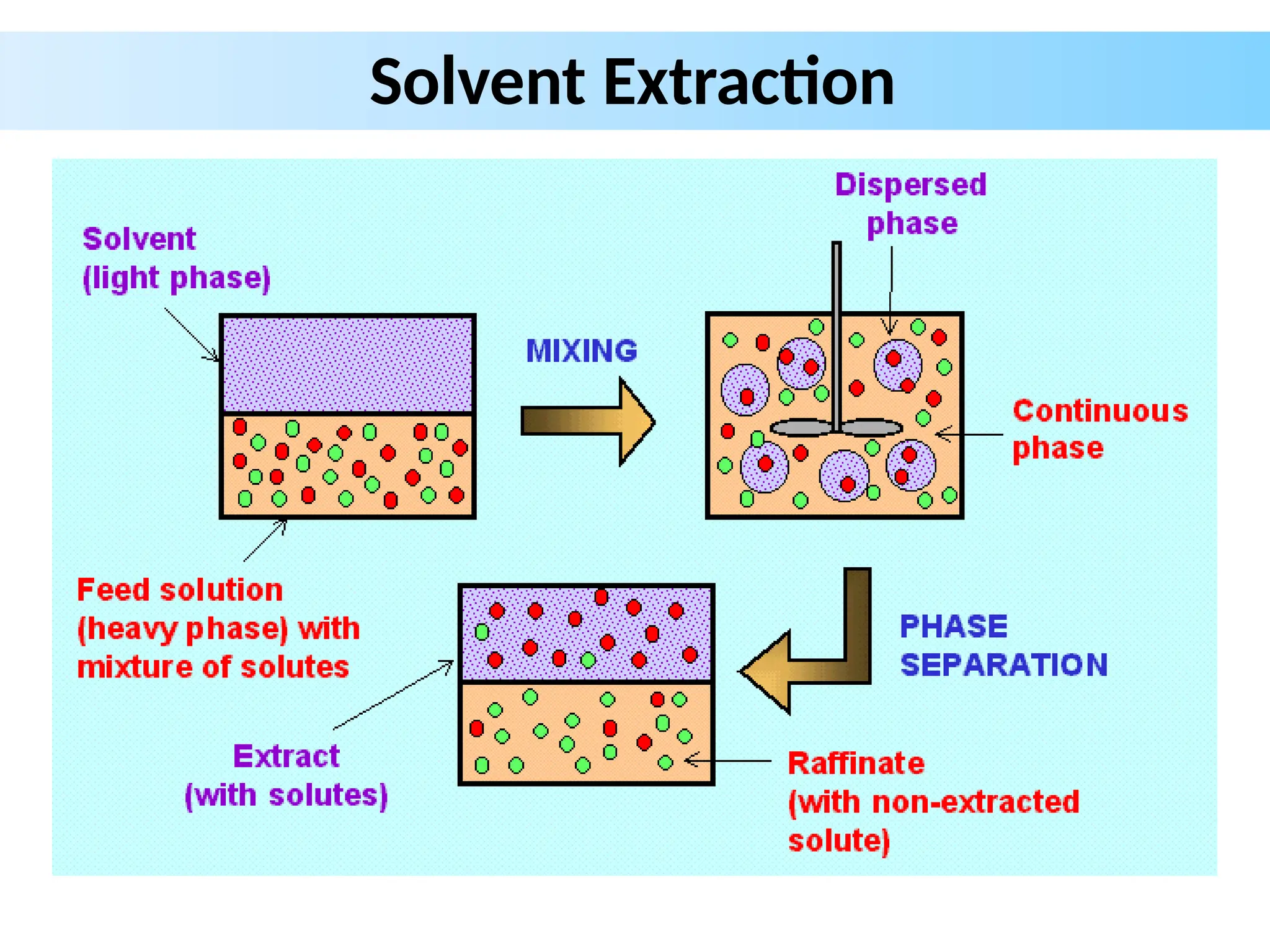
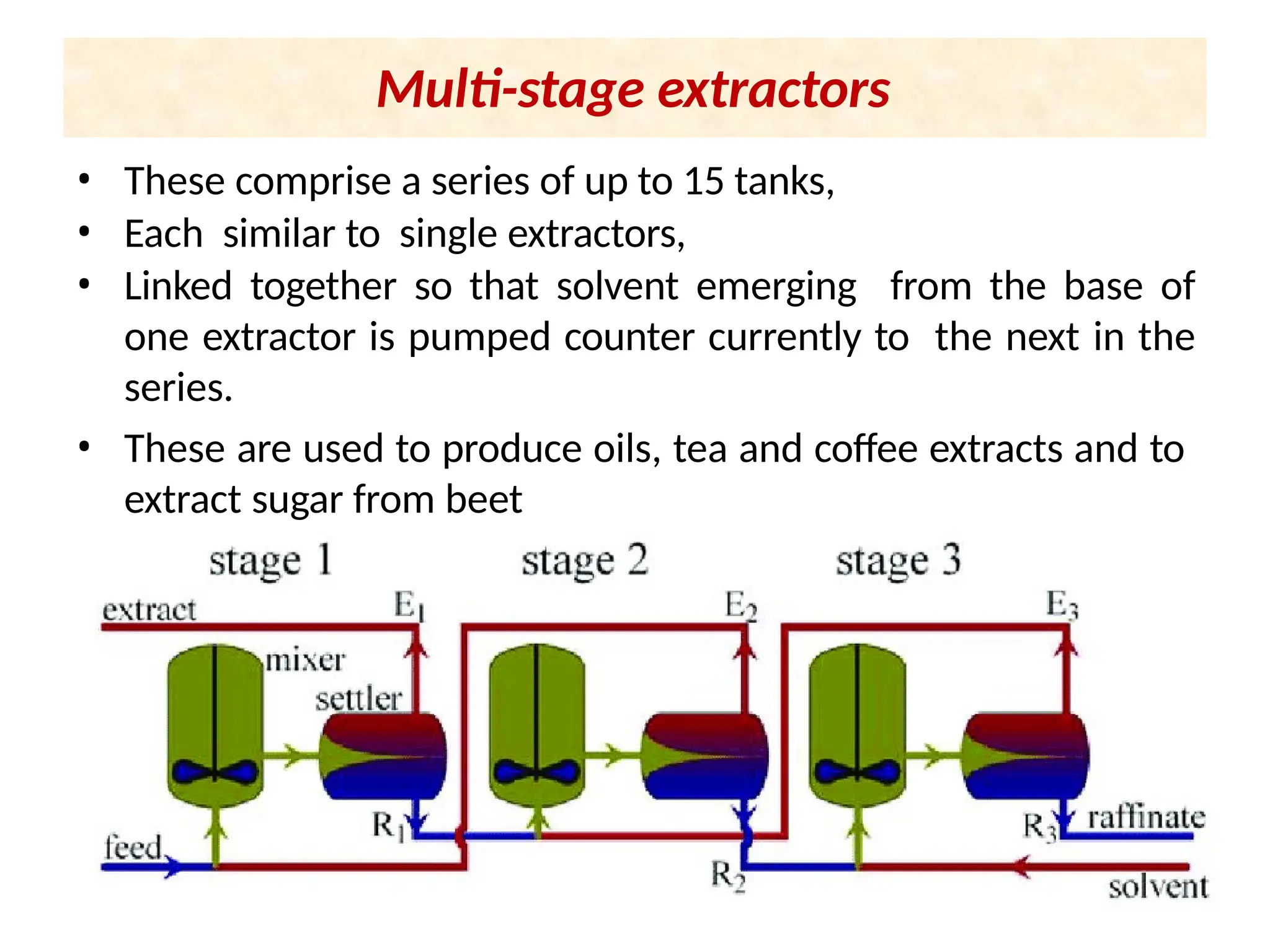
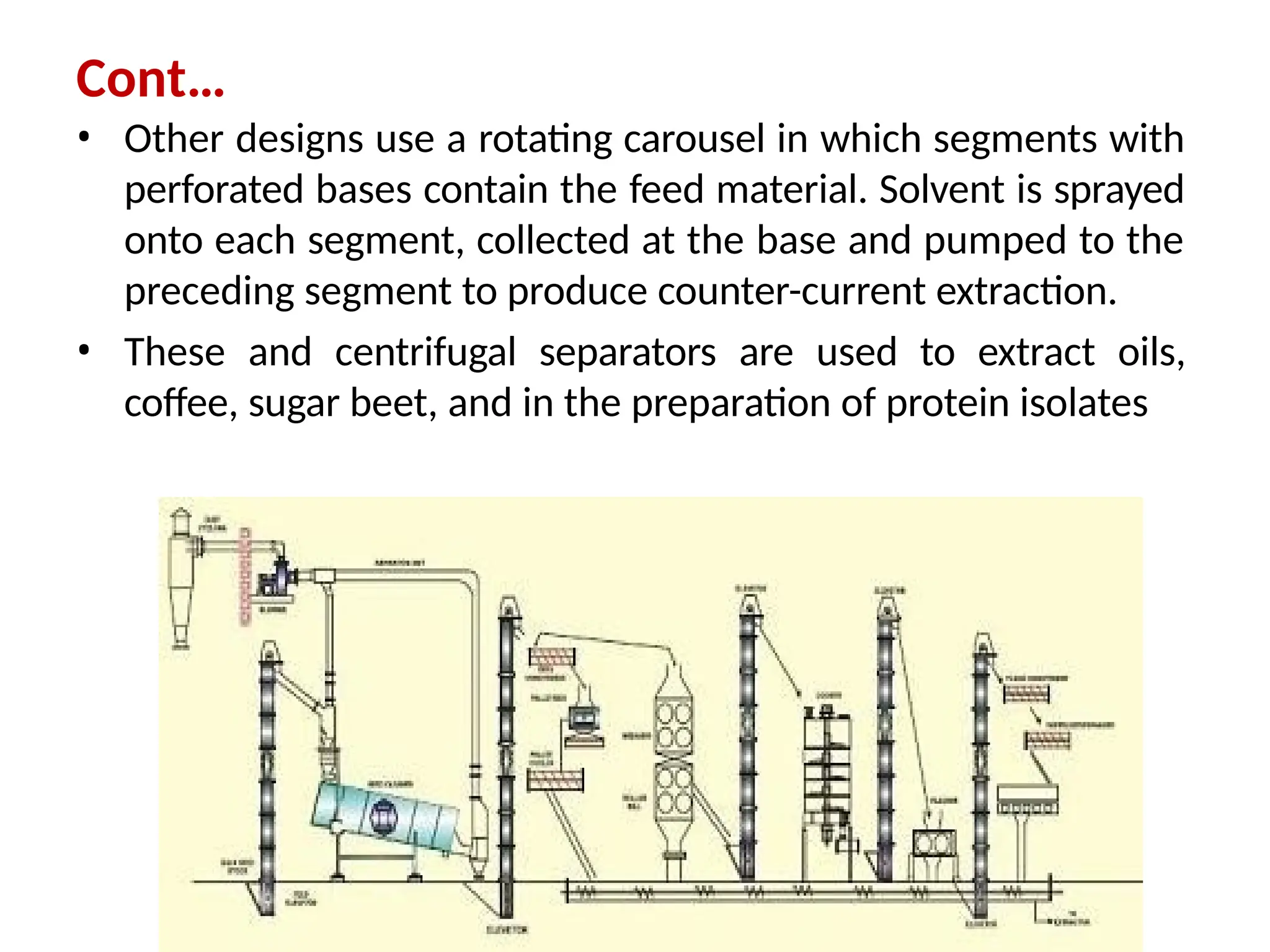
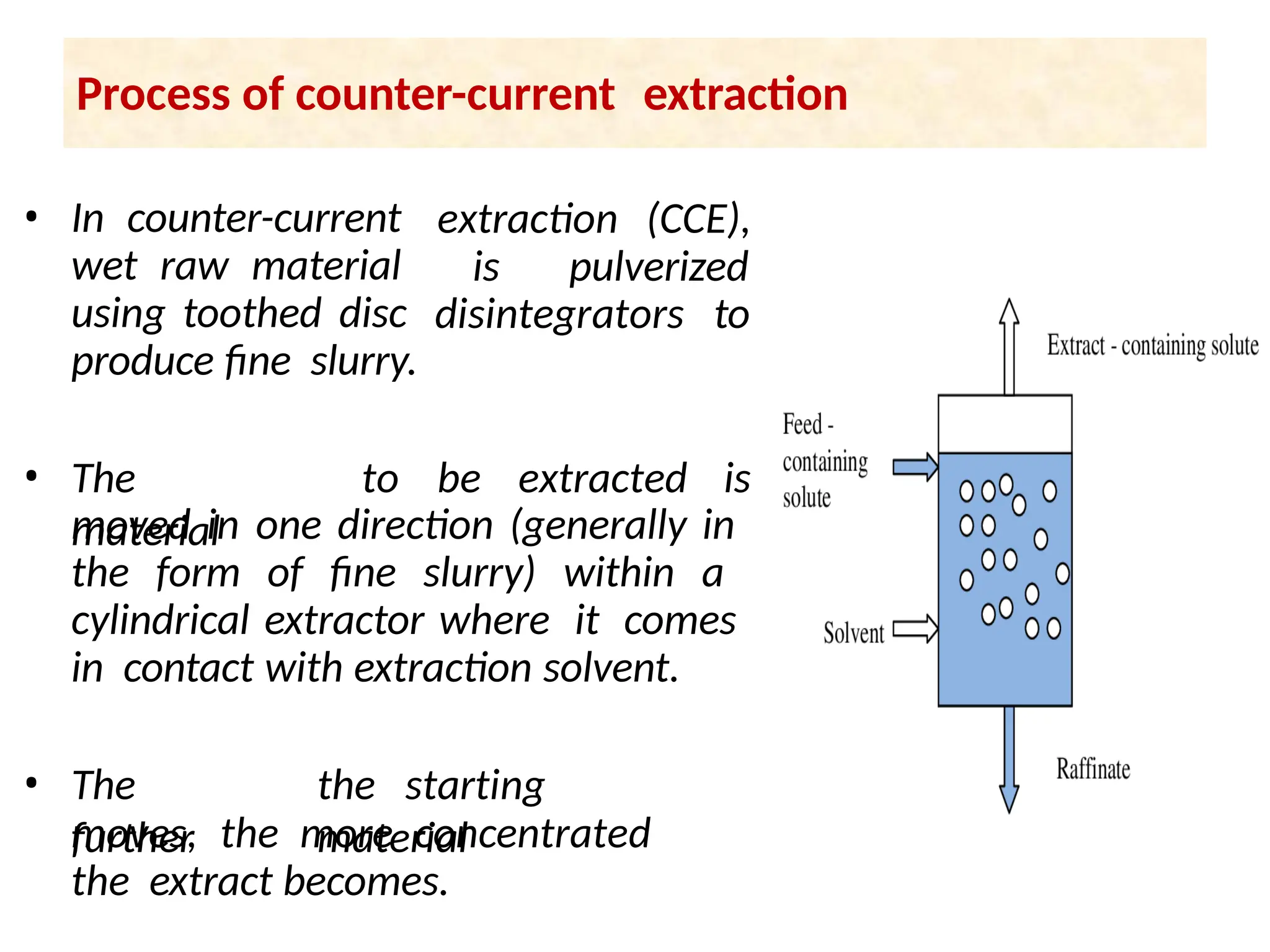
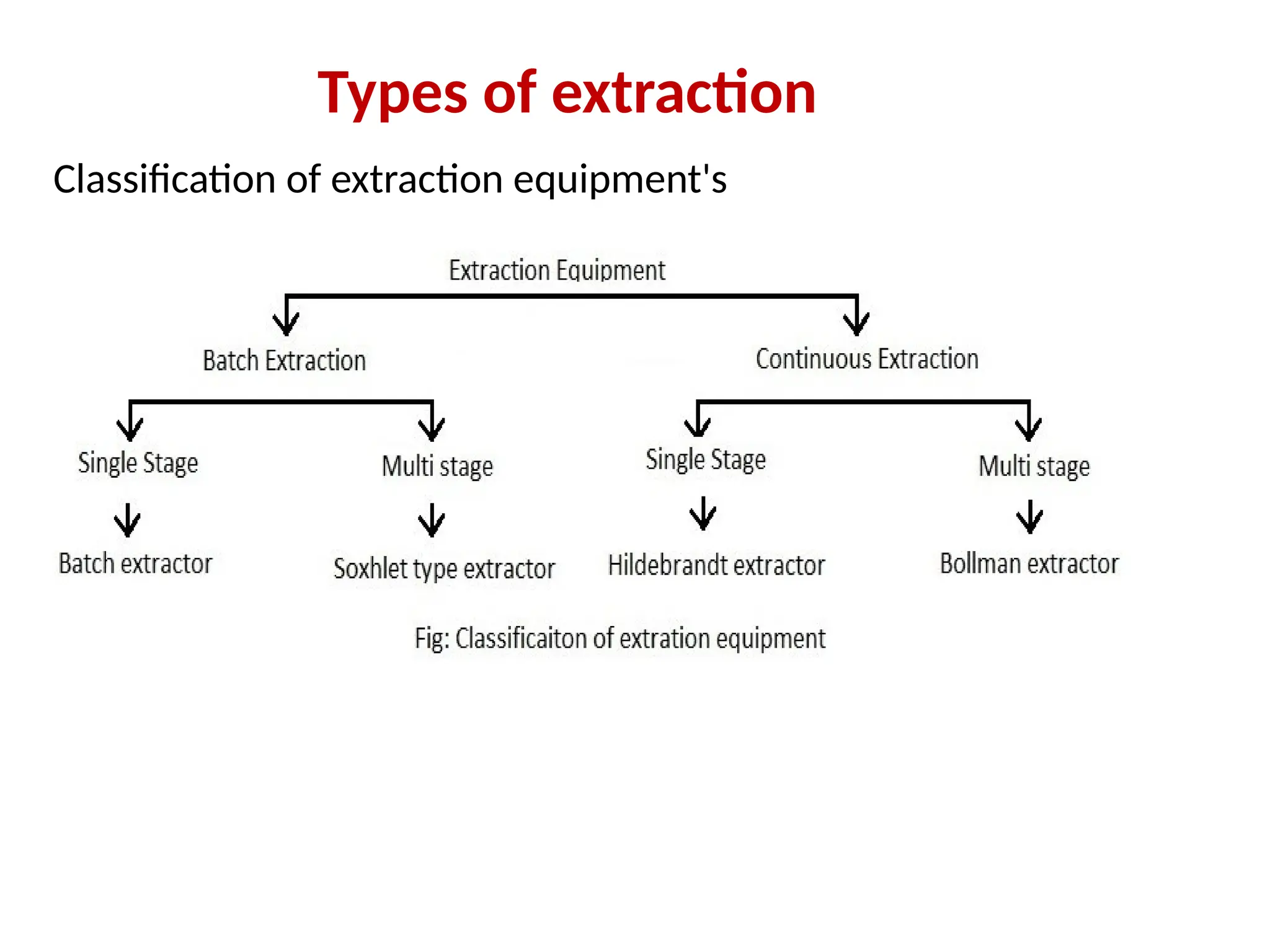
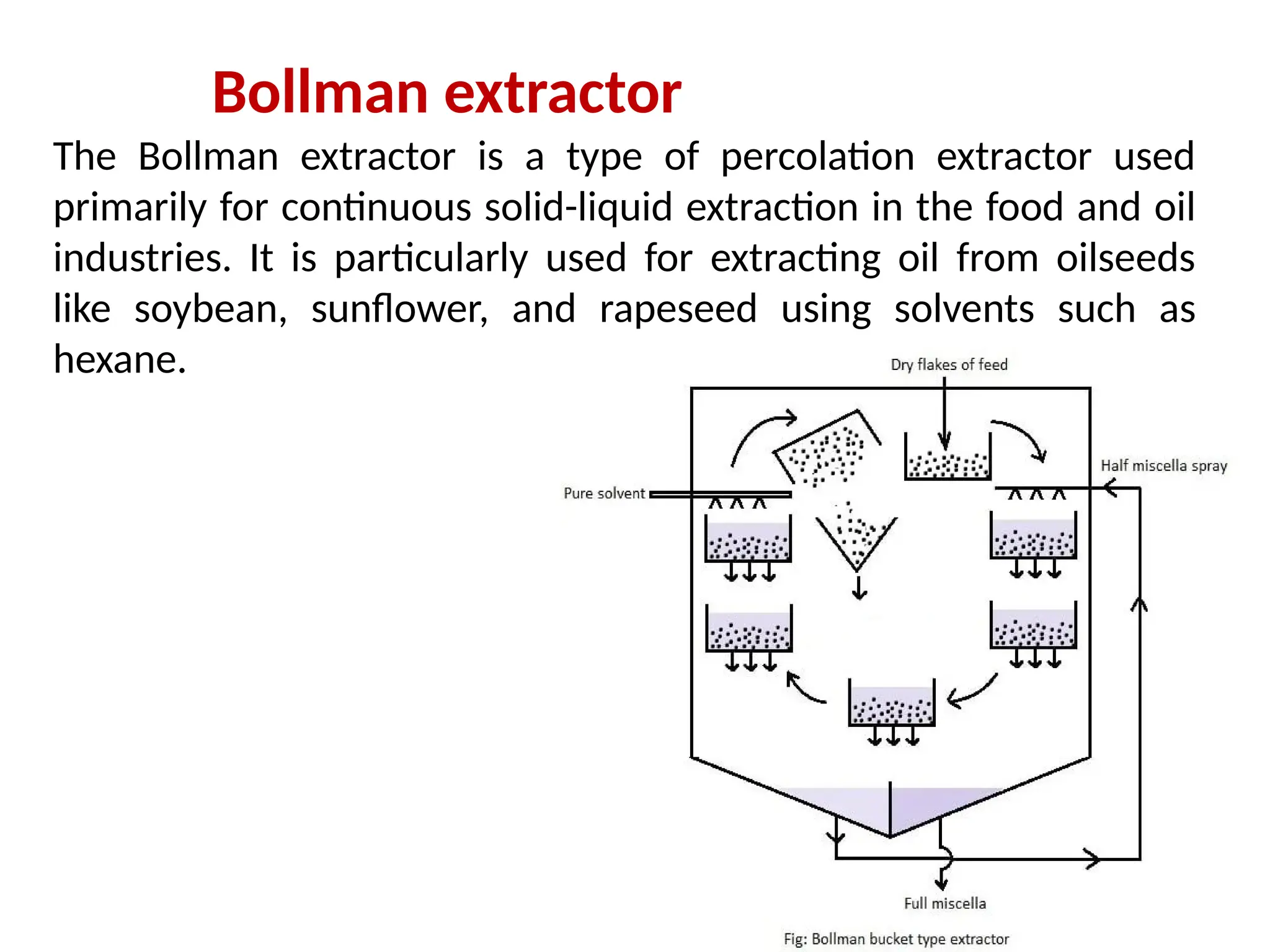
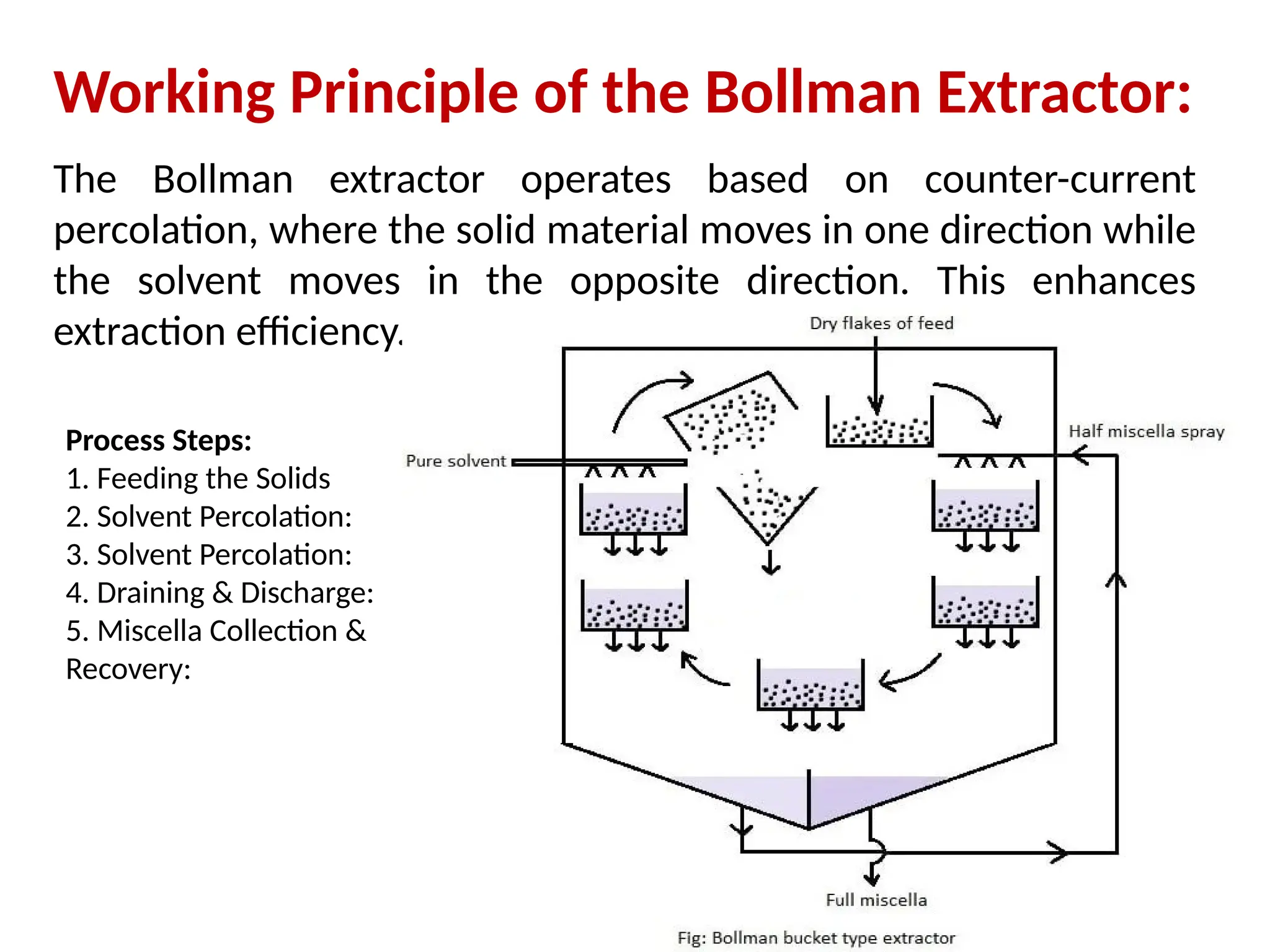
Extraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptxExtraction.pptx