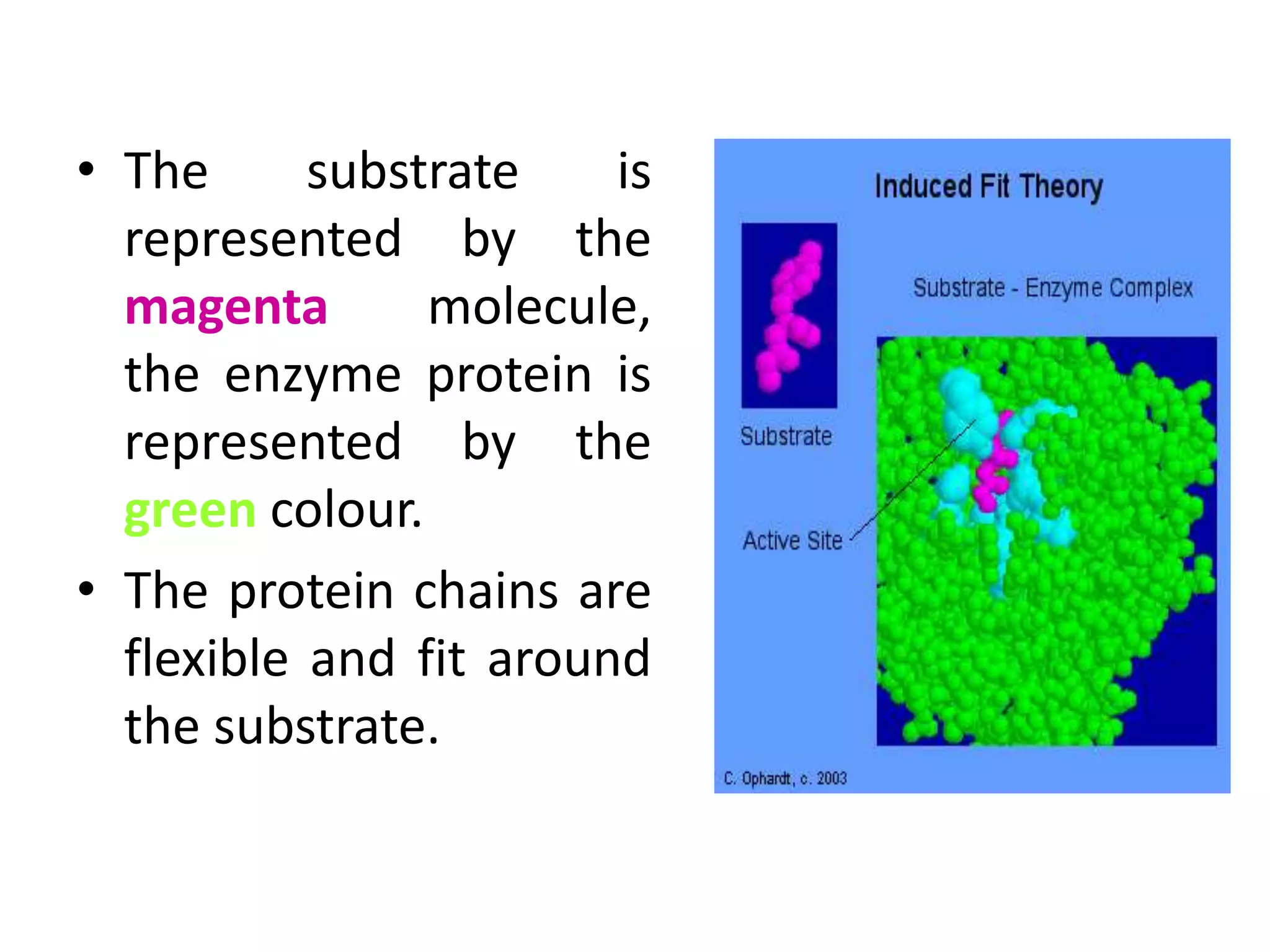
Enzymes are biological catalysts produced by living organisms that speed up chemical reactions without being consumed. They are proteins that contain an active site that binds to specific substrates to catalyze reactions. Enzymes can be classified based on the type of reaction they catalyze or their site of action. Common digestive enzymes include pepsin in the stomach, trypsin in the small intestine, and enzymes from papaya and pineapple such as papain and bromelain which are used to tenderize meat and reduce inflammation.