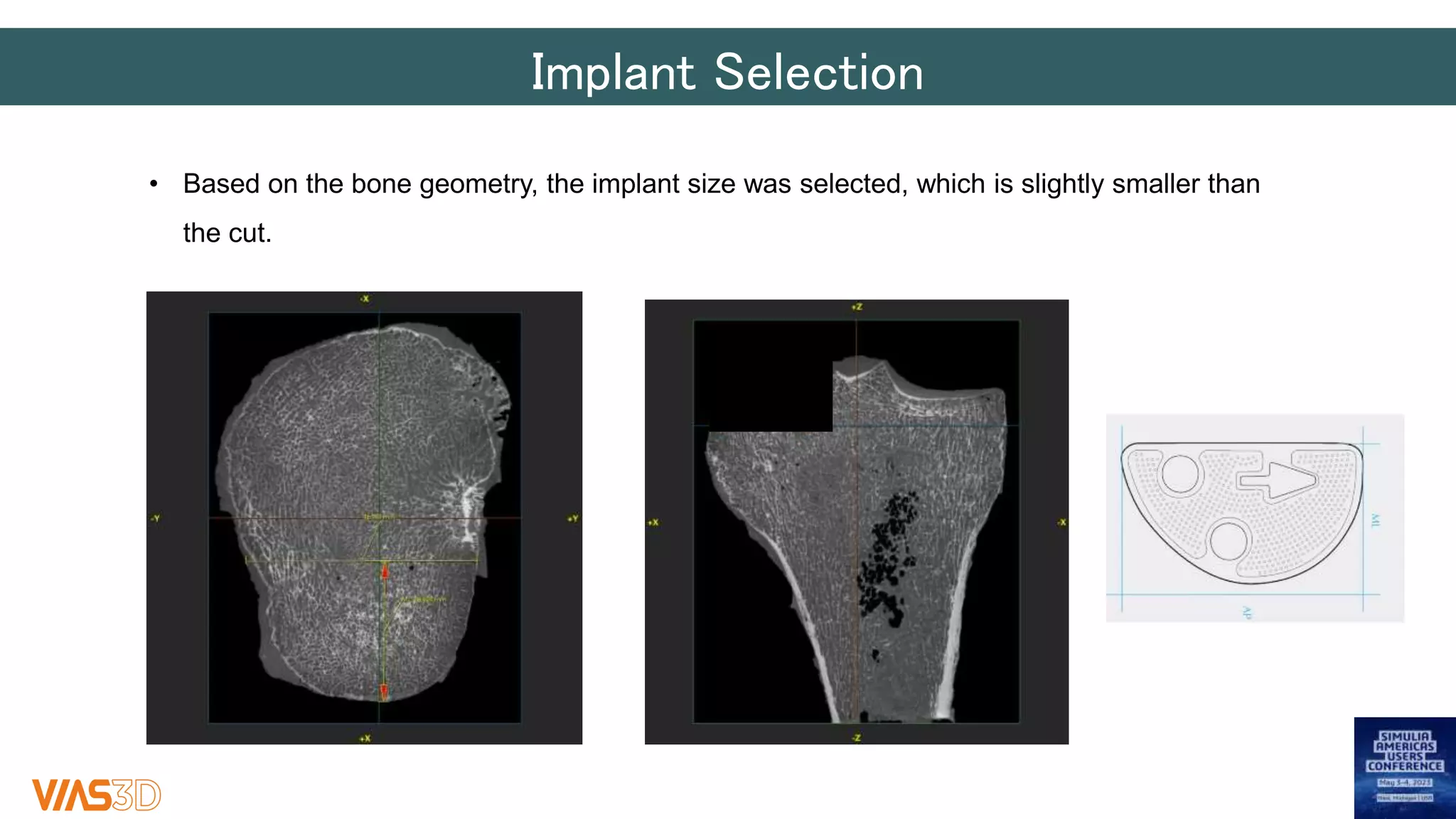
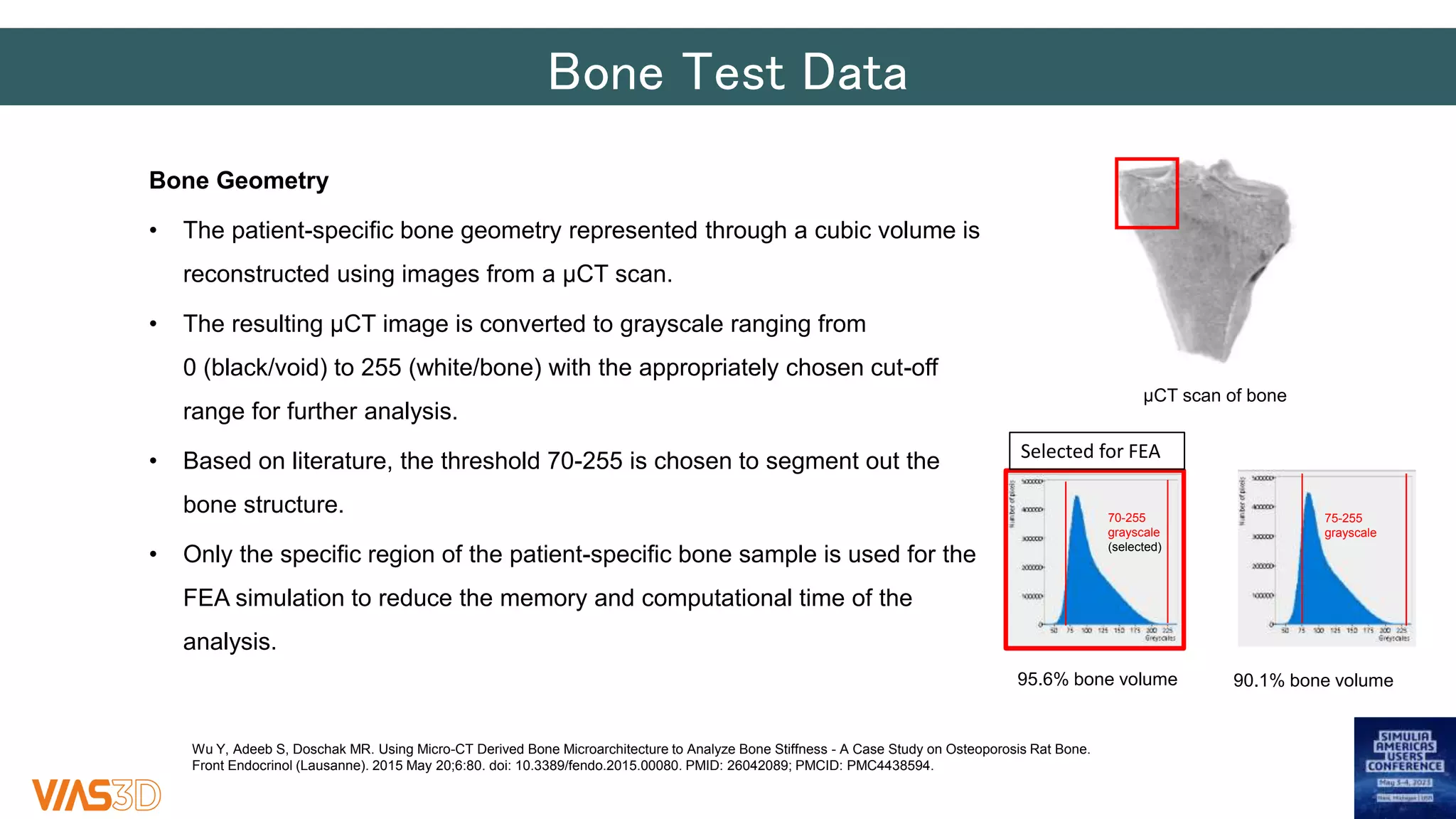
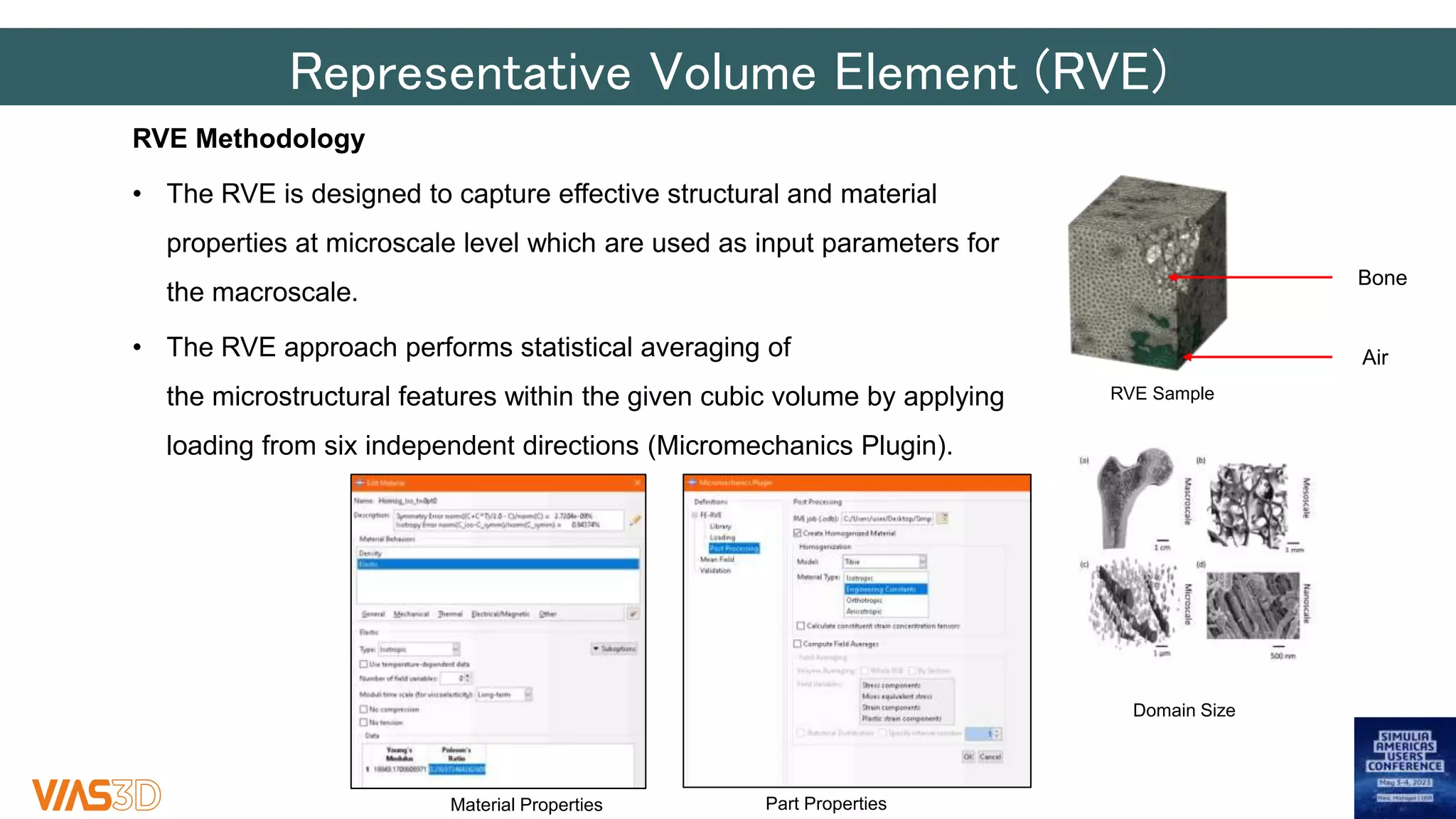
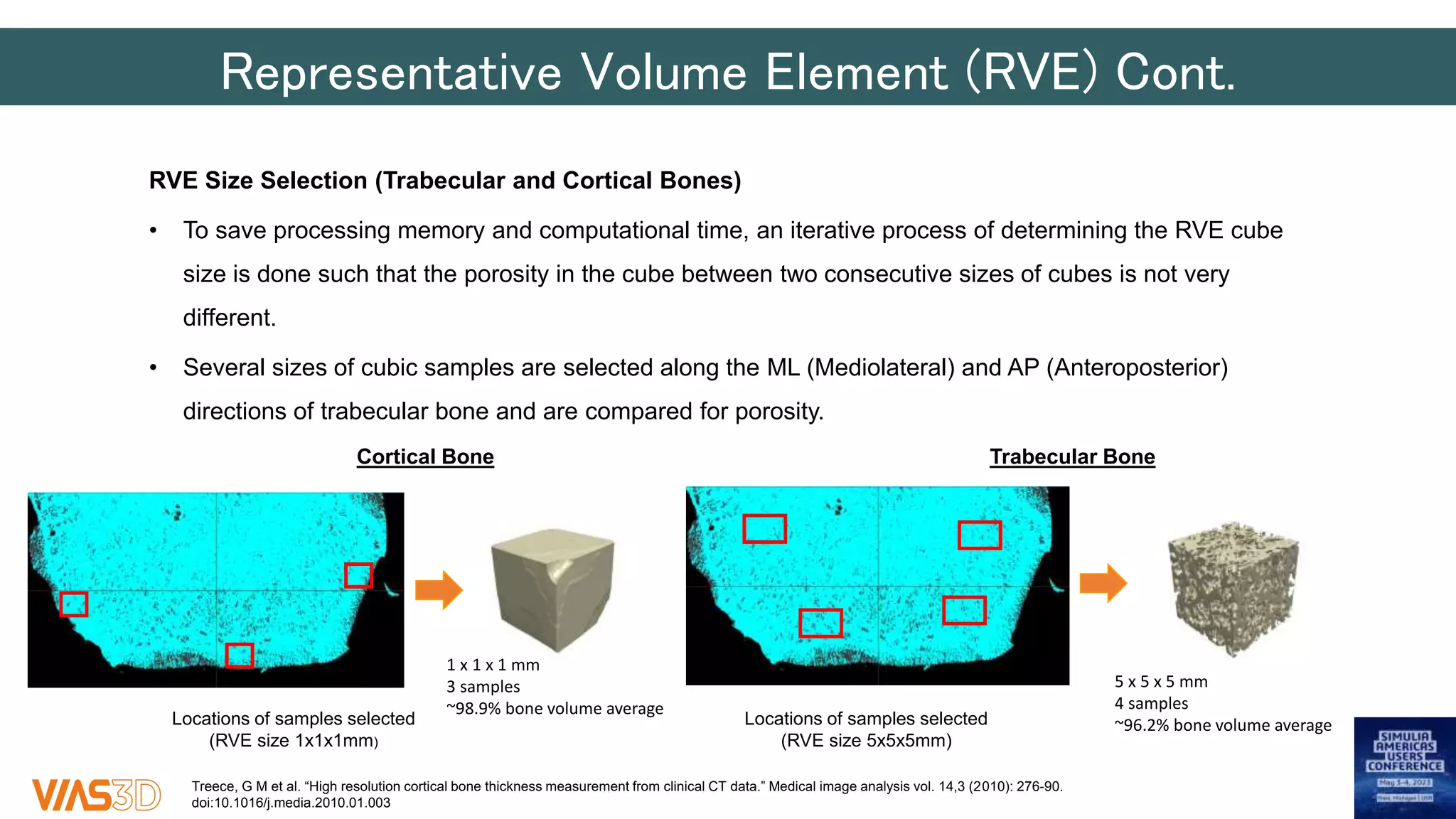
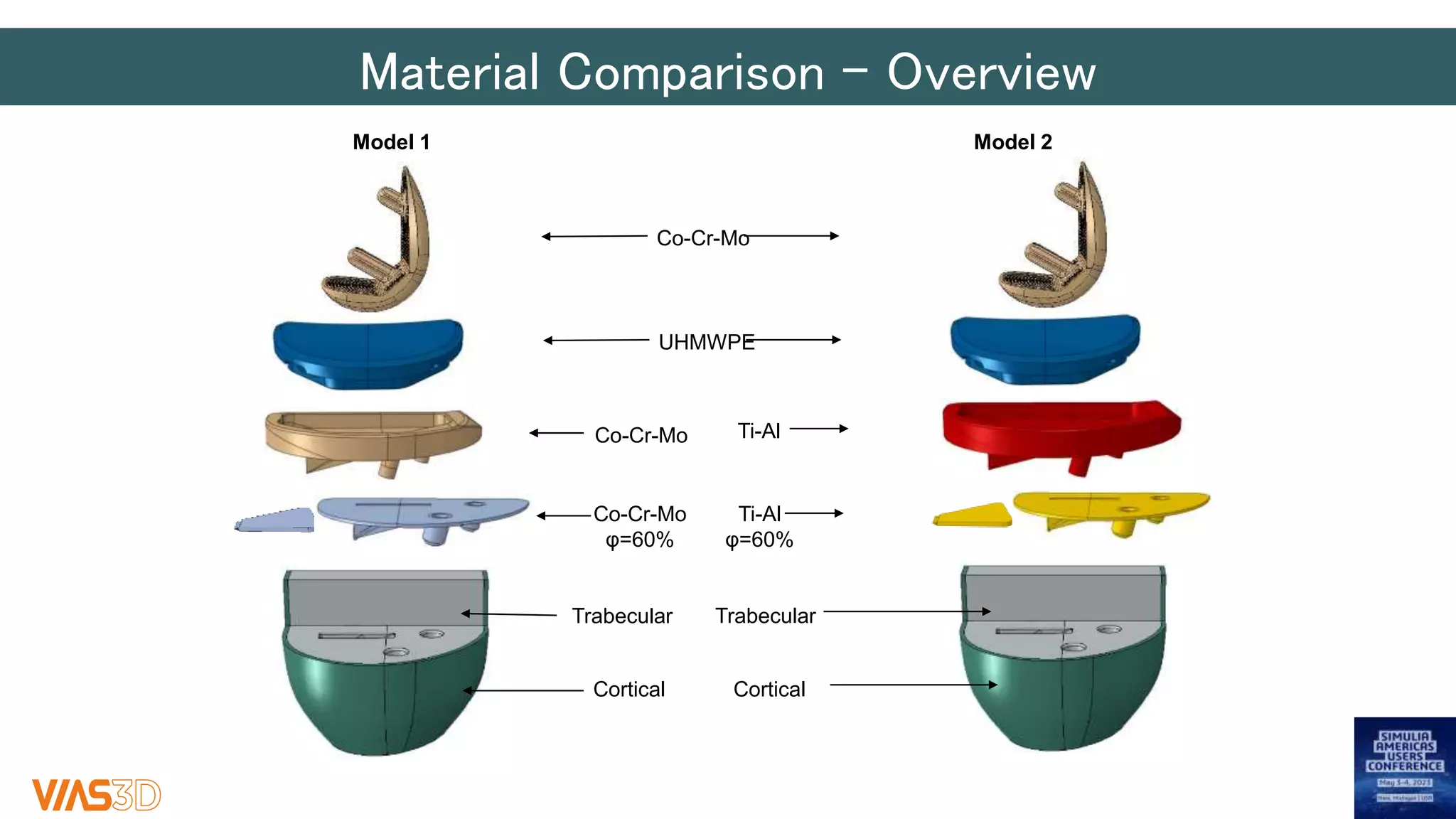
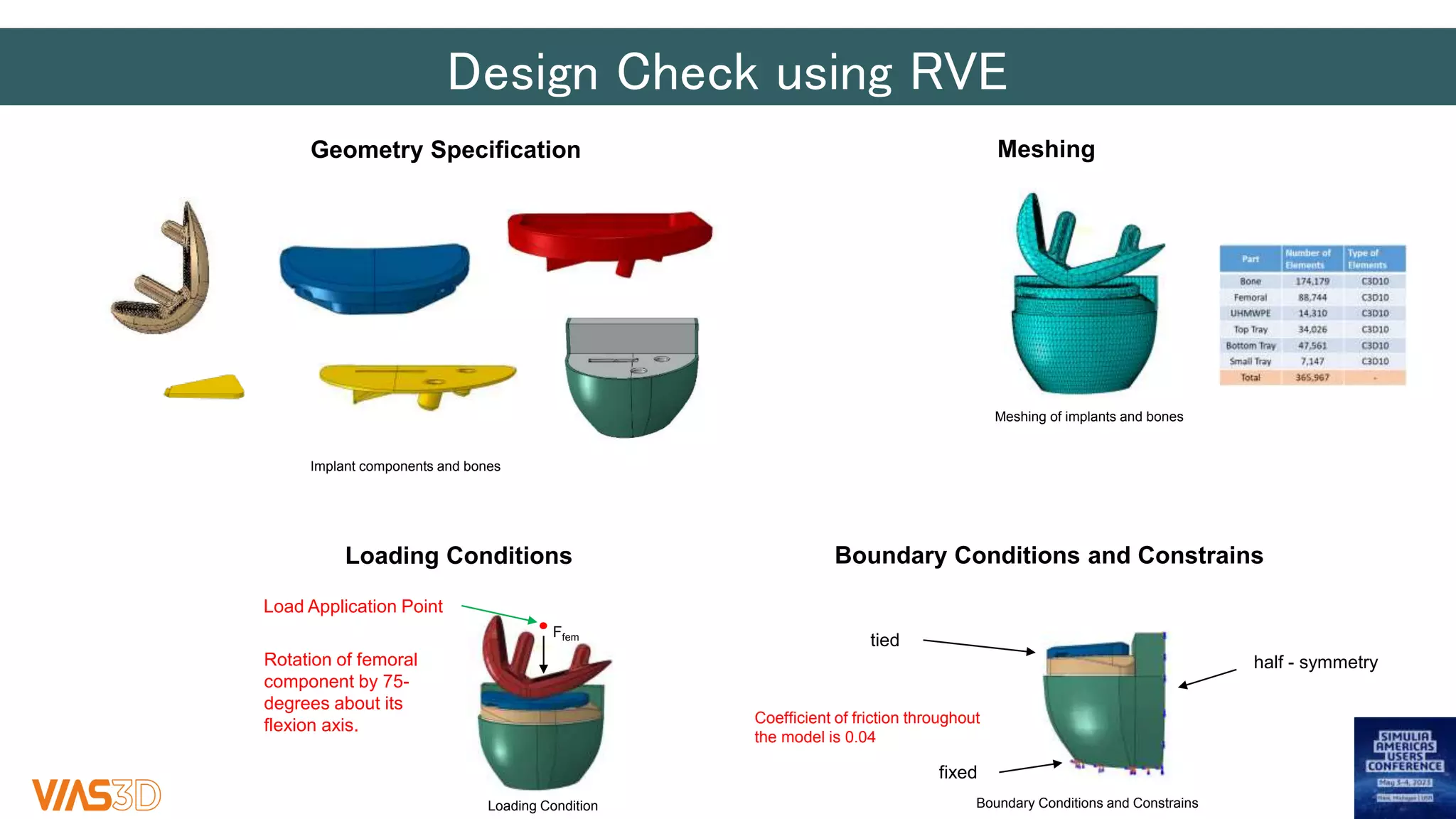
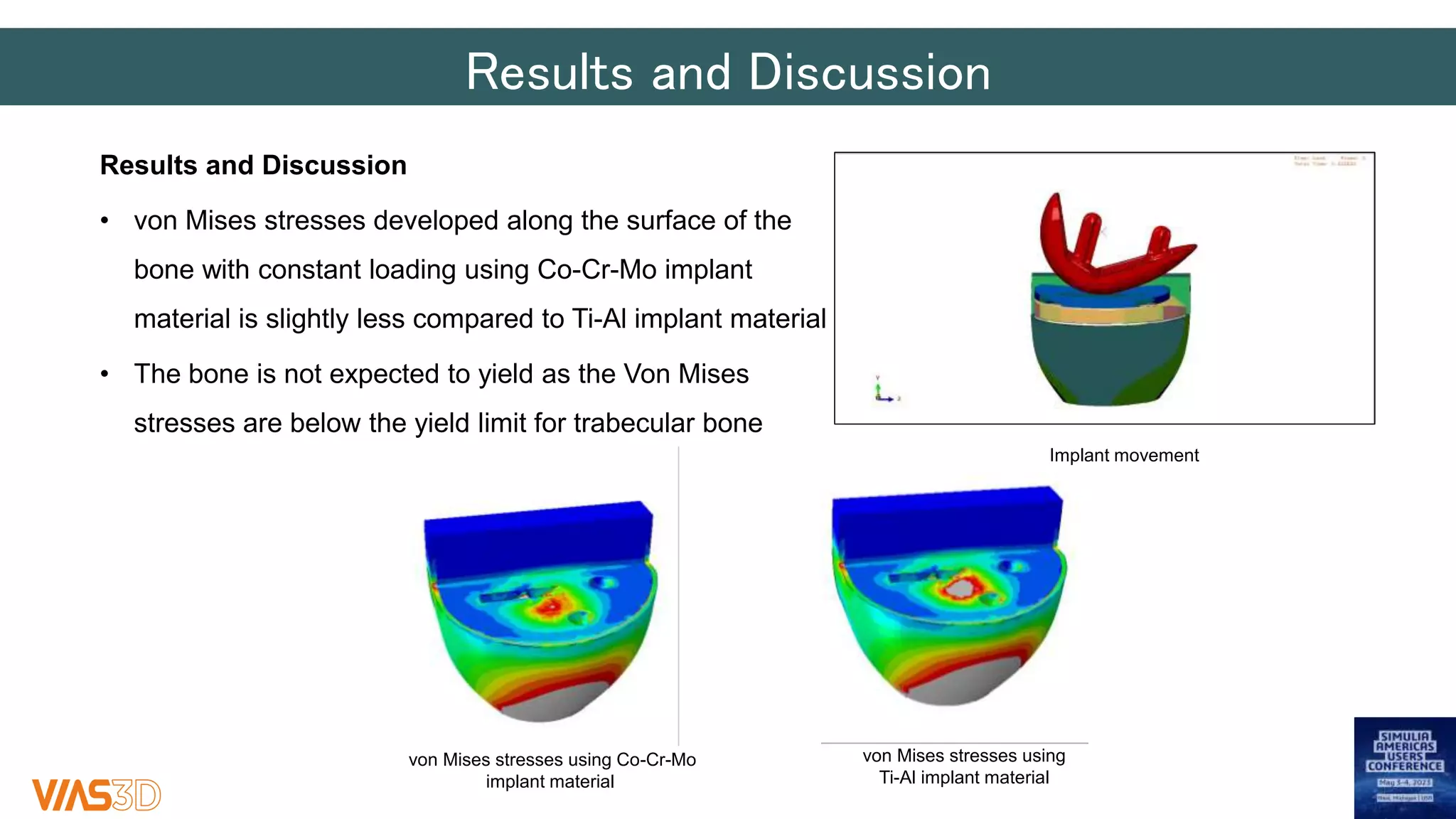
The document discusses the development of patient-specific tibial implants using a micro-macro finite element analysis (FEA) framework. It details the methodology for designing implants based on individual bone geometry obtained from μCT scans and the comparative analysis of different implant materials like Co-Cr-Mo and Ti-Al. The study aims to enhance implant design by leveraging numerical modeling for tailored solutions and reducing material testing and lead times.