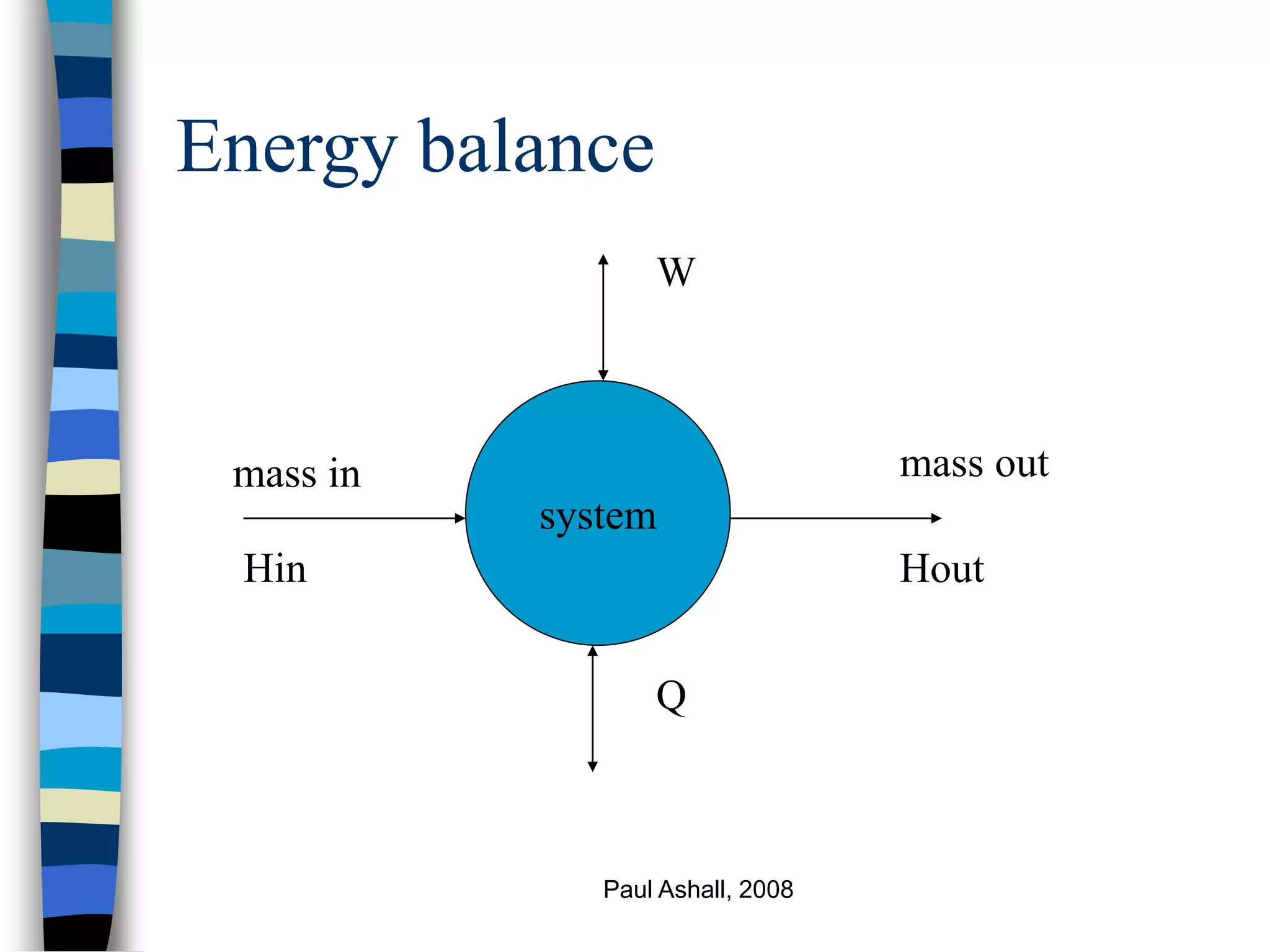
This document discusses energy balances and their application in chemical processes. It covers the first law of thermodynamics, forms of energy, and the conventions used in energy balances. Equations for enthalpy, mechanical energy, and heat capacity are presented. Methods are described for performing energy balances on systems involving phase changes, chemical reactions, and other processes. Key data sources for thermochemical properties are also listed.








![Paul Ashall, 2008
continued
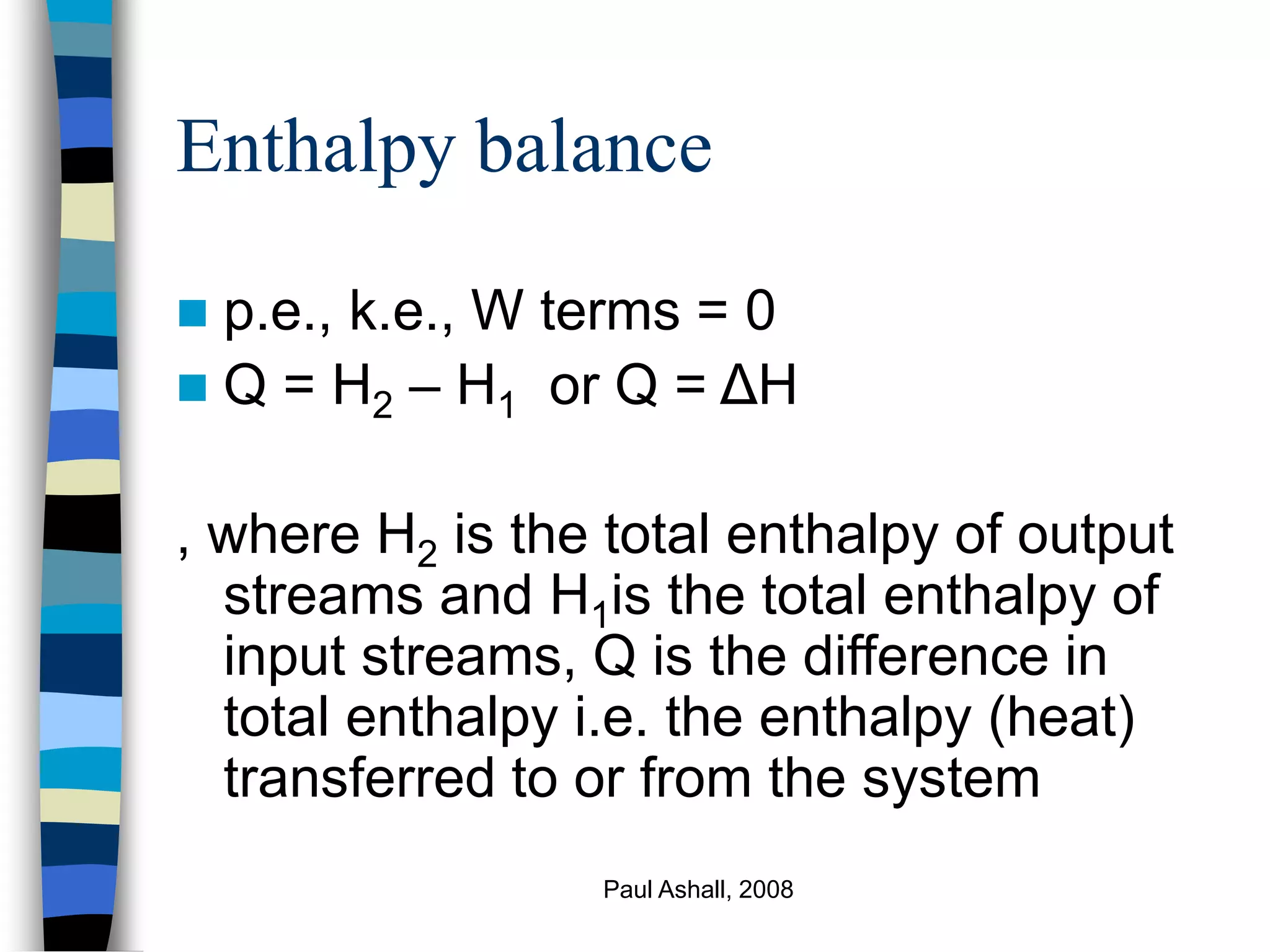
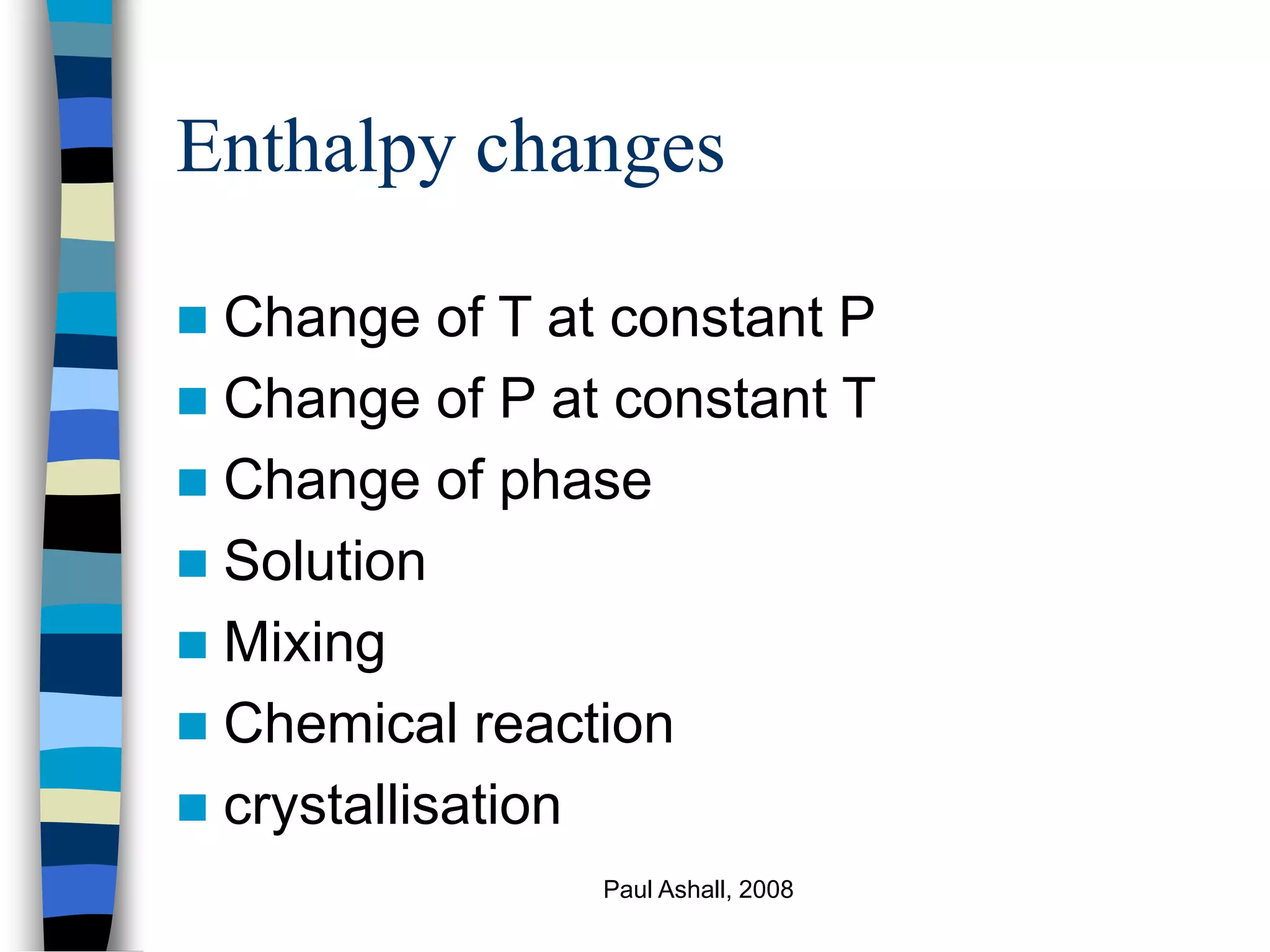
Ignore k.e. and p.e. terms relative to enthalpy
changes for processes involving phase
changes, chemical reactions, large
temperature changes etc
Q = ΔH (enthalpy balance)
Basis for calculation 1 min.
Steady state
Q = Hout – Hin
Q = [295 x 2793.4] – [(120 x 125.7) + (175 x 272)]
Q = + 7.67 x 105 kJ/min](https://image.slidesharecdn.com/energybalance1-221126232345-d41a3aff/75/Energy-Balance-1-ppt-11-2048.jpg)



![Paul Ashall, 2008
continued
Volumetric flow is 20/(1000.60) m3/s
= 0.000333 m3/s
v1 = 0.000333/(π(0.0025)2) = 16.97 m/s
v2 = 0.000333/ (π(0.005)2) = 4.24 m/s
(101325 - P1)/1000 + [(4.24)2 – (16.97)2]/2 + 9.81.50 = 0
P1 = 456825 Pa (4.6 bar)](https://image.slidesharecdn.com/energybalance1-221126232345-d41a3aff/75/Energy-Balance-1-ppt-18-2048.jpg)
![Paul Ashall, 2008
continued
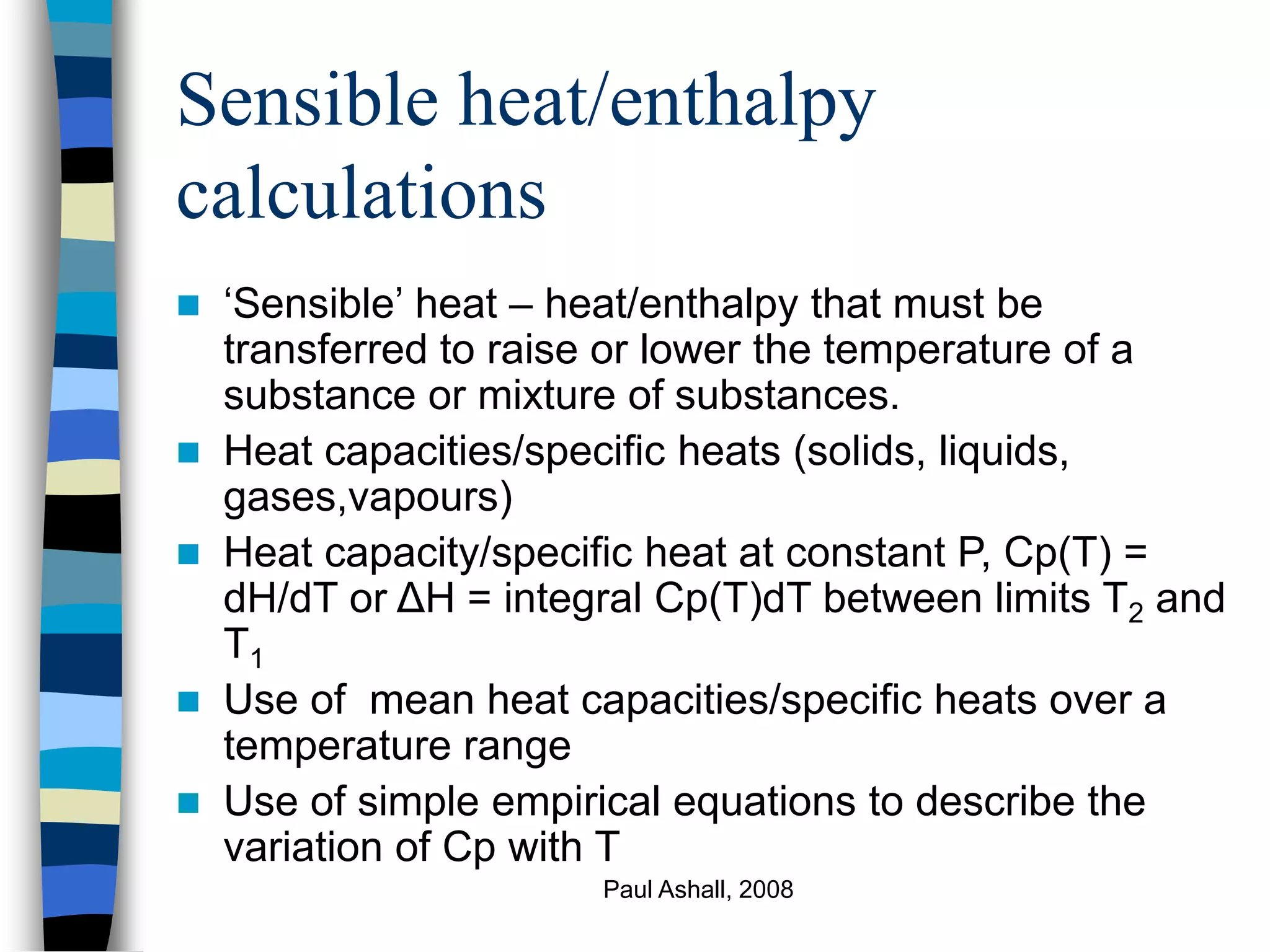
e.g. Cp = a + bT + cT2 + dT3
,where a, b, c, d are coefficients
ΔH = integralCpdT between limits T2, T1
ΔH = [aT + bT2 + cT3 + dT4]
2 3 4
Calculate values for T = T2, T1 and subtract
Note: T may be in deg cent or K - check units for Cp!](https://image.slidesharecdn.com/energybalance1-221126232345-d41a3aff/75/Energy-Balance-1-ppt-20-2048.jpg)


![Paul Ashall, 2008
Example – change of phase
A feed stream to a distillation unit contains an equimolar
mixture of benzene and toluene at 10 deg cent.The
vapour stream from the top of the column contains
68.4 mol % benzene at 50 deg cent. and the liquid
stream from the bottom of the column contains 40
mol% benzene at 50 deg cent.
[Need Cp (benzene, liquid), Cp (toluene, liquid), Cp
(benzene, vapour), Cp (toluene, vapour), latent heat
of vapourisation benzene, latent heat of vapourisation
toluene.]](https://image.slidesharecdn.com/energybalance1-221126232345-d41a3aff/75/Energy-Balance-1-ppt-23-2048.jpg)

![Paul Ashall, 2008
Enthalpy balance equation -
reactor
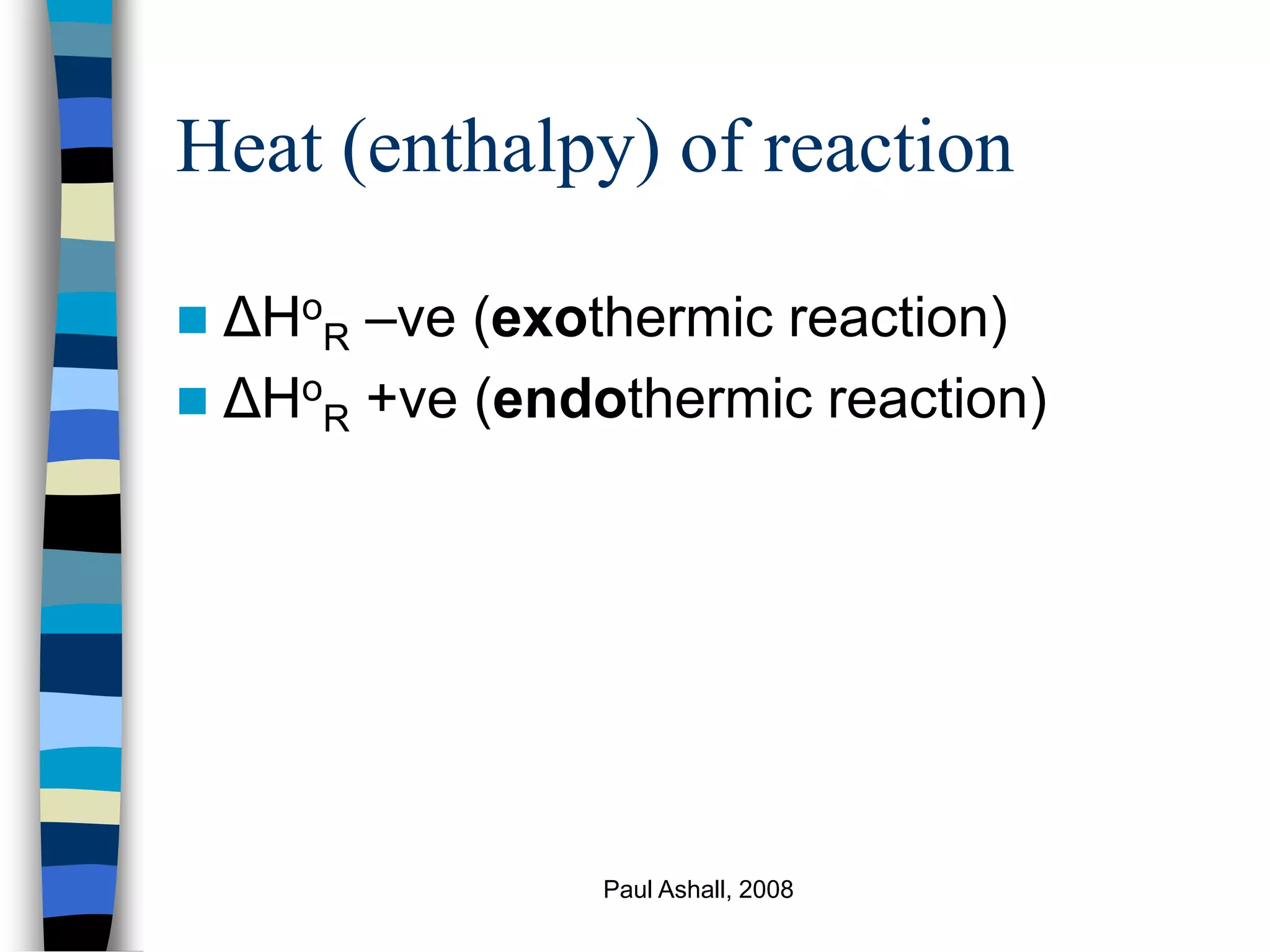
Qp = Hproducts – Hreactants + Qr
Qp – heat transferred to or from process
Qr – reaction heat (ζ ΔHo
R), where ζ is
extent of reaction and is equal to [moles
component,i, out – moles component i,
in]/ νi](https://image.slidesharecdn.com/energybalance1-221126232345-d41a3aff/75/Energy-Balance-1-ppt-26-2048.jpg)