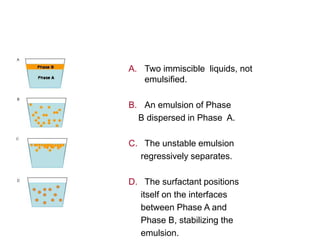
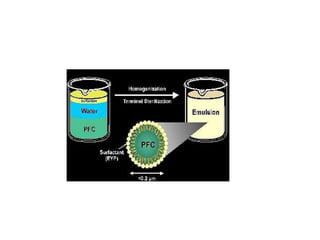
The document discusses emulsions, which are two-phase systems composed of immiscible liquids, with a focus on their stability and applications in pharmacy. It details the roles of emulsifying agents, the mechanisms of instability including flocculation, creaming, and breaking, and methods for preparing emulsions. The advantages of emulsions include masking unpleasant tastes, enhancing solubility and stability of drugs, and offering improved absorption for various dosage forms.