1. The document discusses the wave and particle nature of light and provides evidence from phenomena such as interference, diffraction for the wave nature and the photoelectric effect and Compton effect for the particle nature.
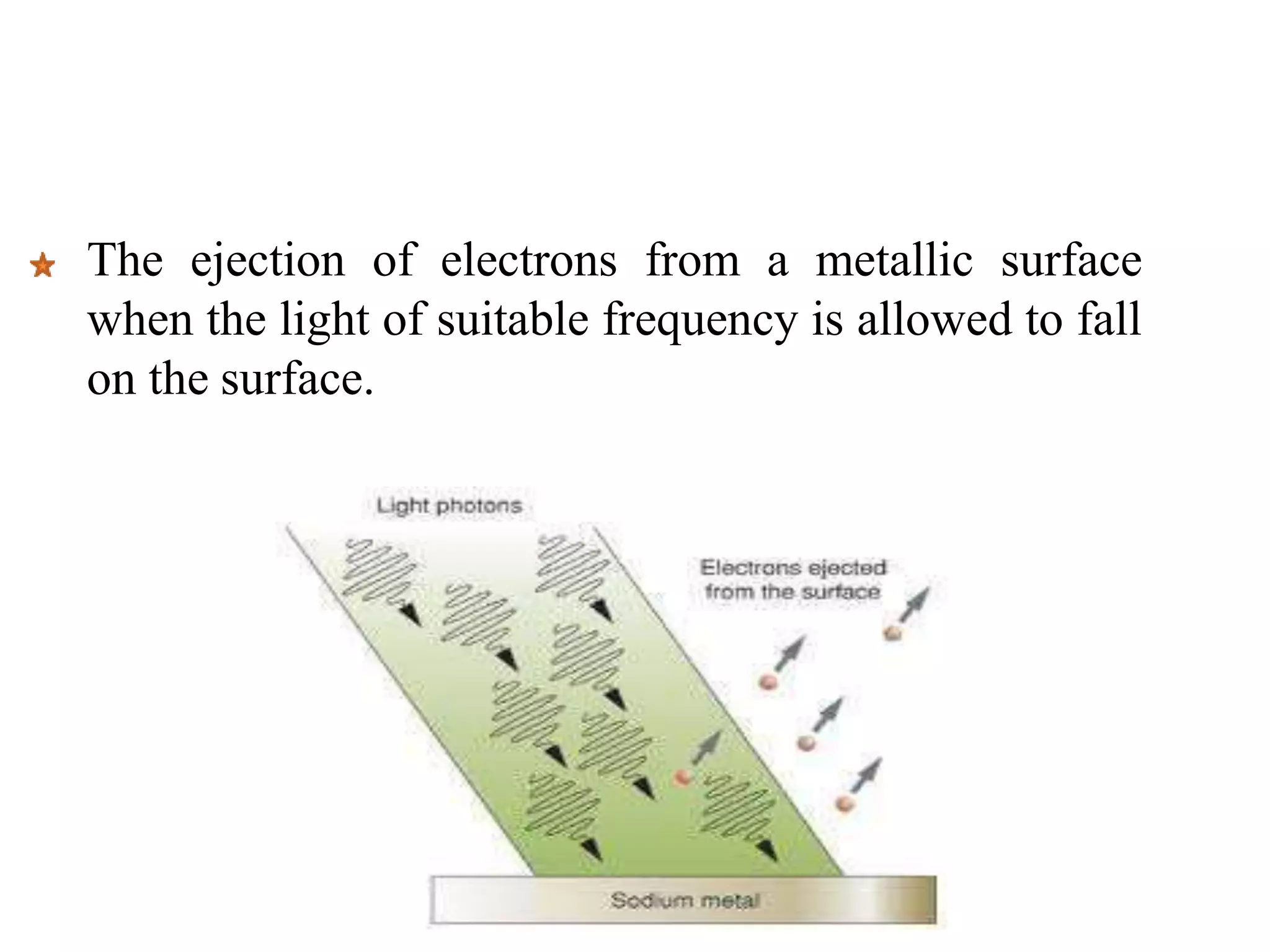
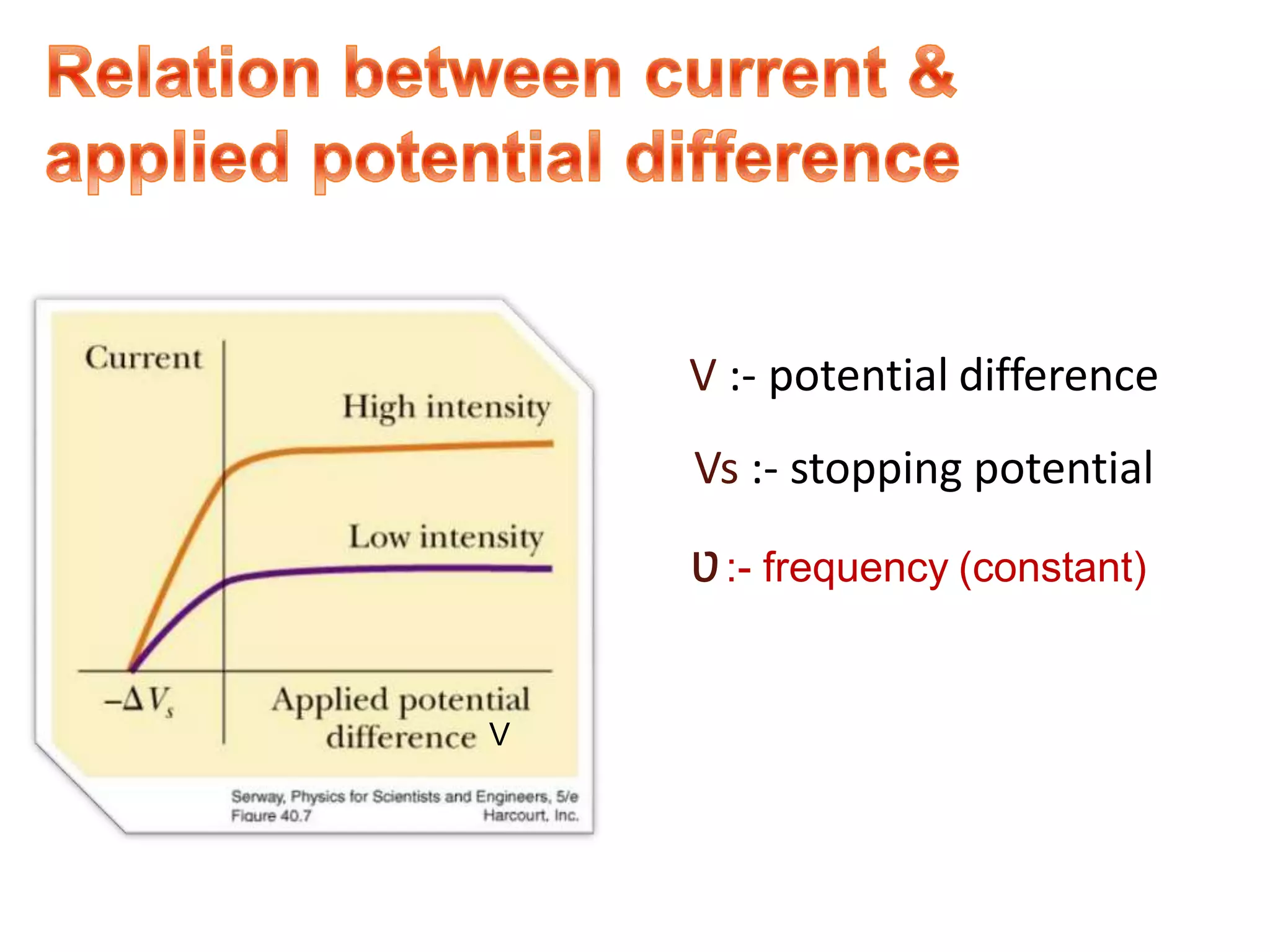
2. It then describes the photoelectric effect in detail, explaining terms like threshold frequency, work function, and how Einstein's photoelectric equation explained the instantaneous emission of electrons.
3. Applications of the photoelectric effect include its use in cameras for light meters and in security systems.