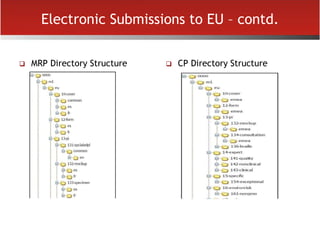
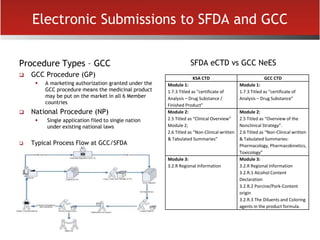
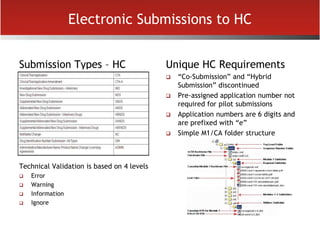
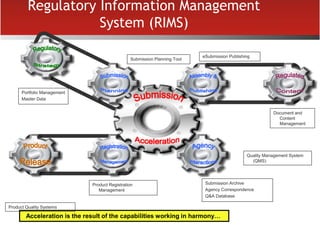
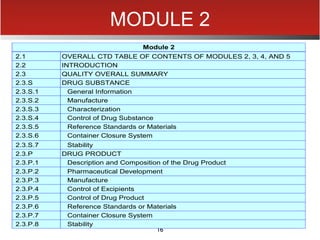
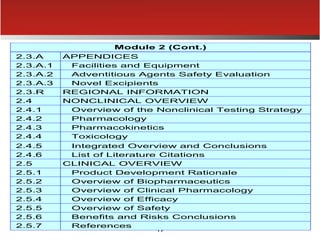
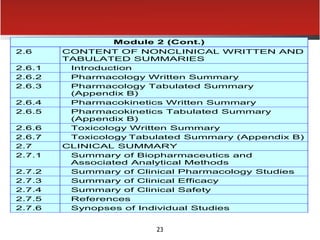
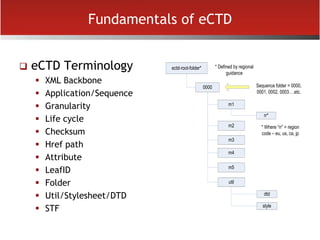
The document provides an overview of electronic Common Technical Document (eCTD) format for regulatory submissions globally. It defines key terms related to eCTD and describes the history and structure of the Common Technical Document (CTD) format adopted by the International Conference on Harmonization (ICH). The document compares paper, non-eCTD electronic and eCTD submission formats and highlights benefits of eCTD format. It also provides high-level information on eCTD fundamentals, folder structure and backbone XML view. Finally, it discusses key requirements and considerations for electronic submissions to major regulatory authorities in US, EU, Saudi Arabia, GCC countries and Health Canada.

























![Electronic Submissions to EU
Procedure Types – EU
Centralised Procedure (CP)
A marketing authorisation granted under the
centralised procedure means the medicinal
product may be put on the market in all
Member States
Decentralised Procedure (DCP)
Use if application will be made to several
member states and product has not received
marketing authorization in any member state
at time of application
Mutual Recognition Procedure (MRP)
Use if application will be made to several
member states and product has received
marketing authorization in any member state
at time of application
National Procedure (NP)
Single application filed to single nation
under existing national laws
Unique EU Sub Requirements
Country Specific Envelope(s)
“Common” directory for files common to
all nations receiving the submission
File naming as per the pattern [country
code]-[document type code]-[variable
component].[file type extension]
Tracking Table mandatory for MRP and DCP
Submissions
Node Extensions allowed, especially in the
case of Module 5
Product Information has both country and
language designations
“To be Advised” can be used if procedure
number not provided by the RMS or NCA
Unique Agency Codes and Names
Drop Box facility available for submitting
dossiers online](https://image.slidesharecdn.com/ectd-230821055557-03147833/85/eCTD-pptx-35-320.jpg)