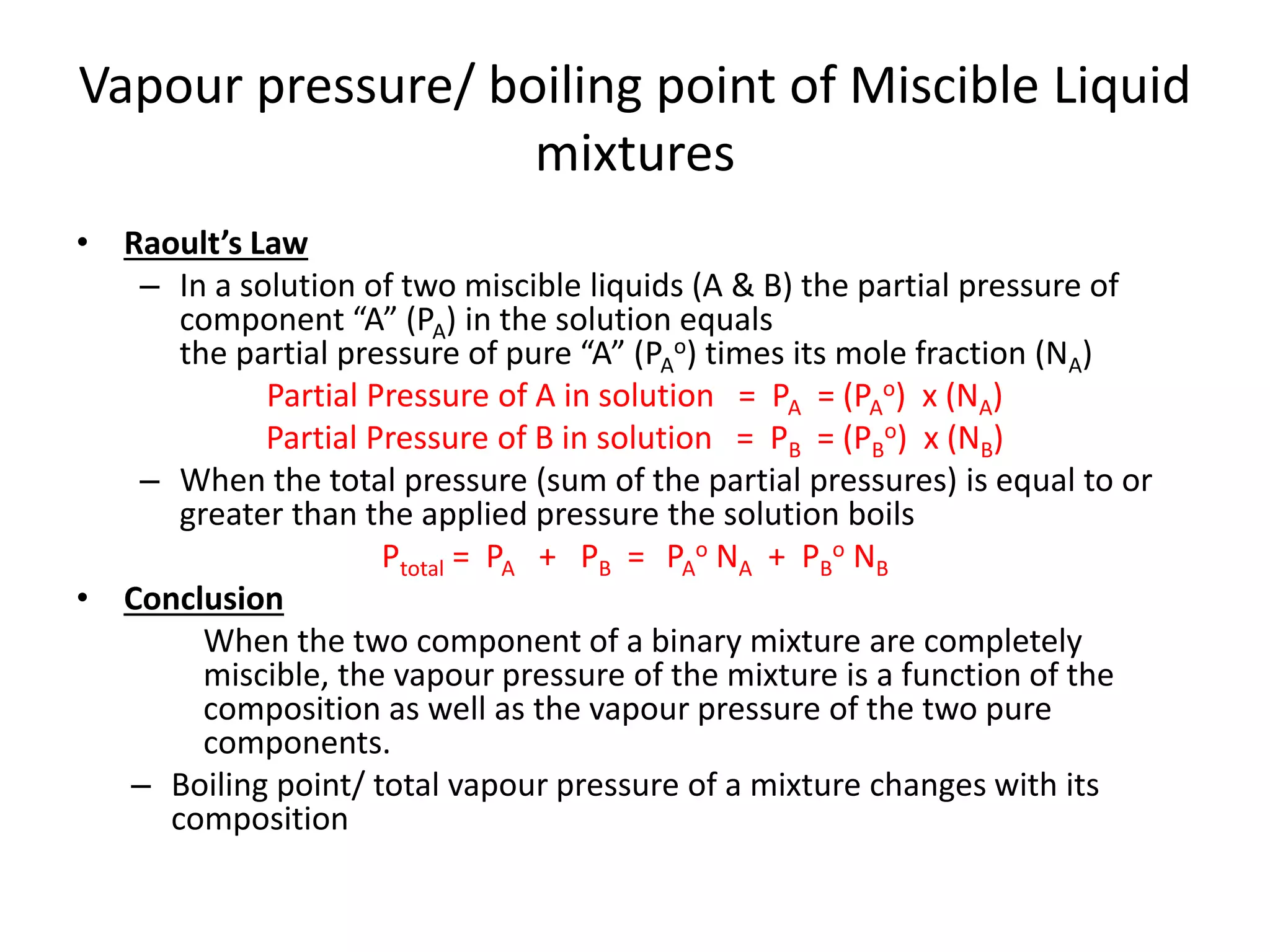
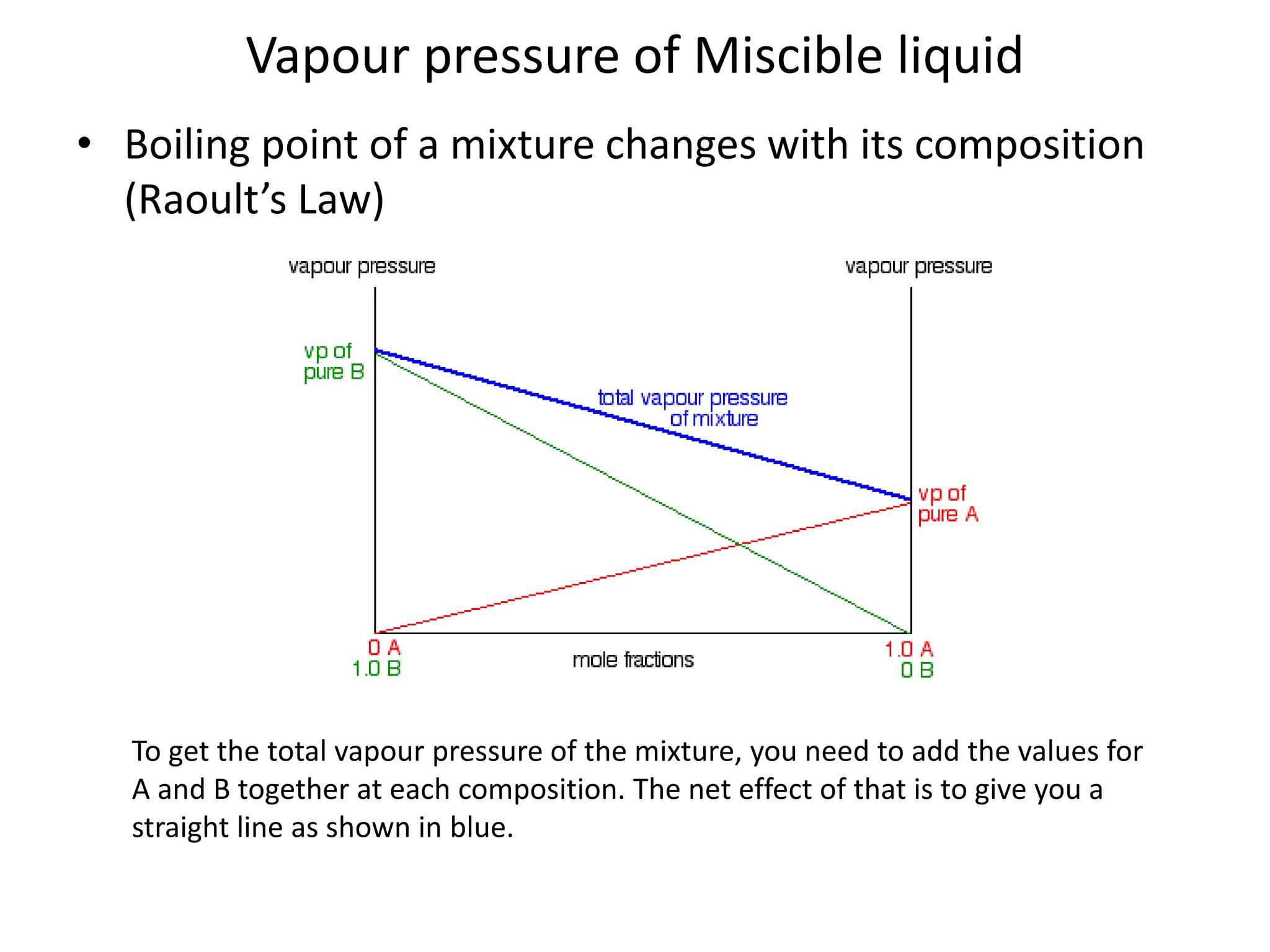
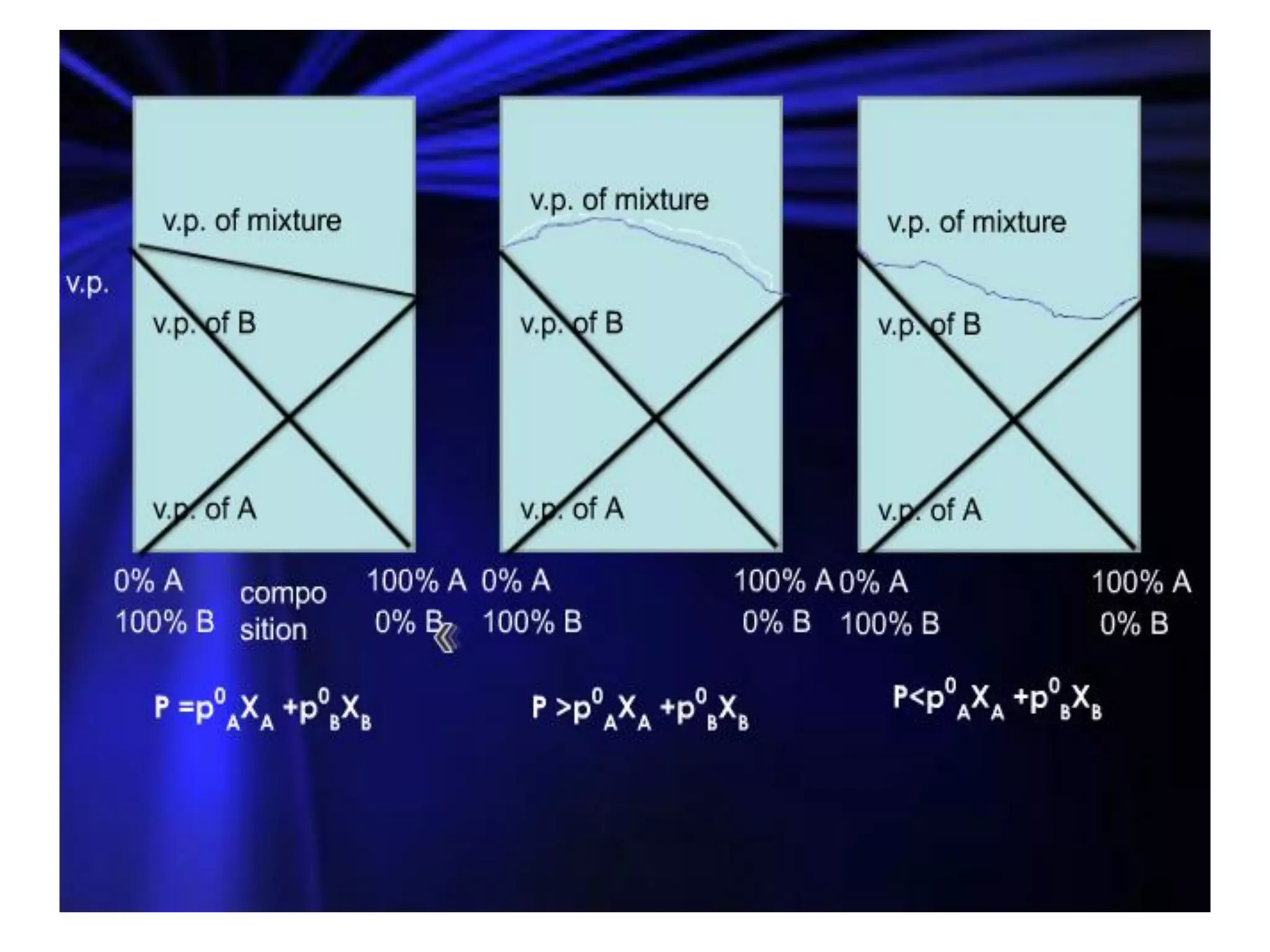
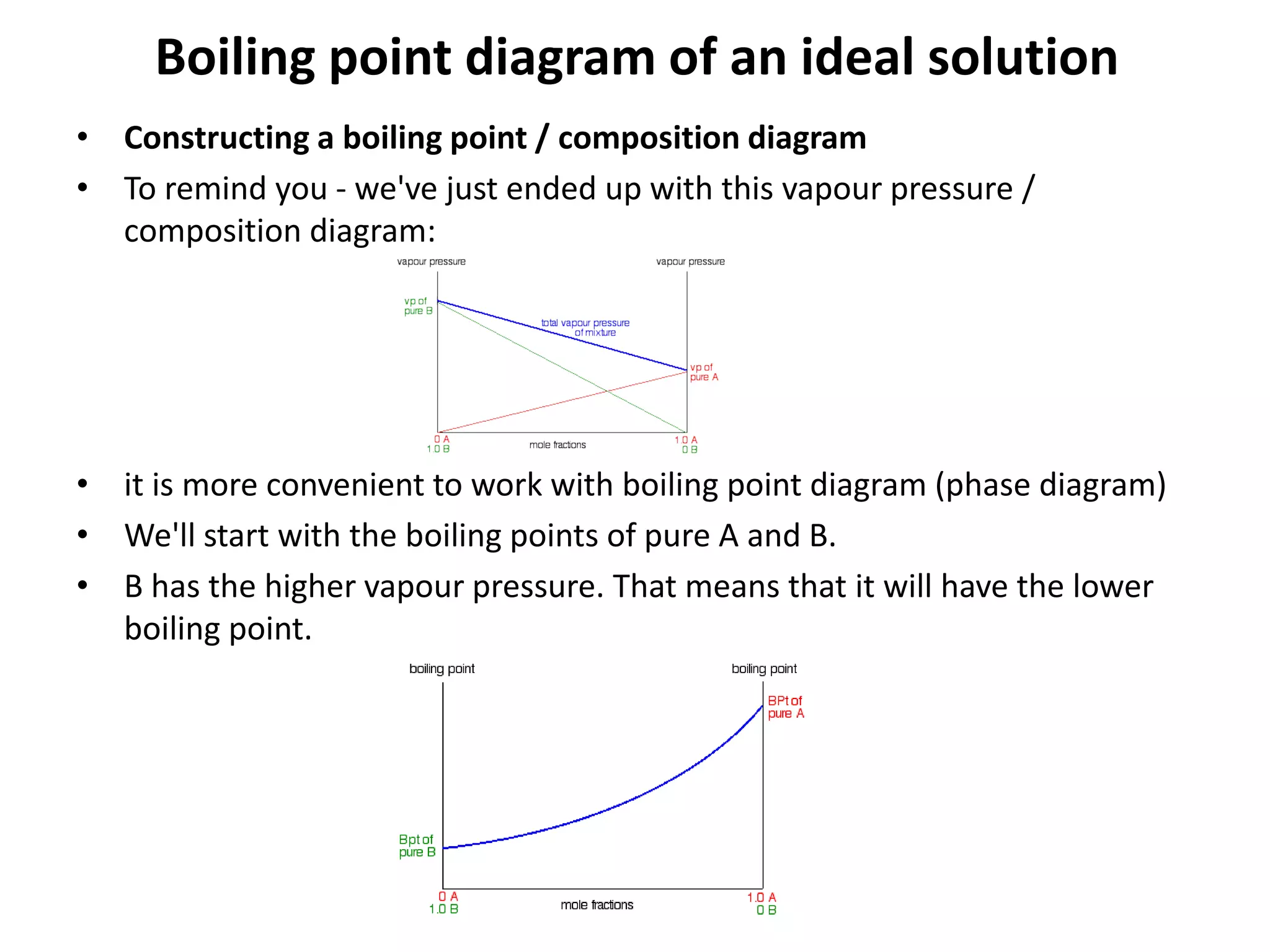
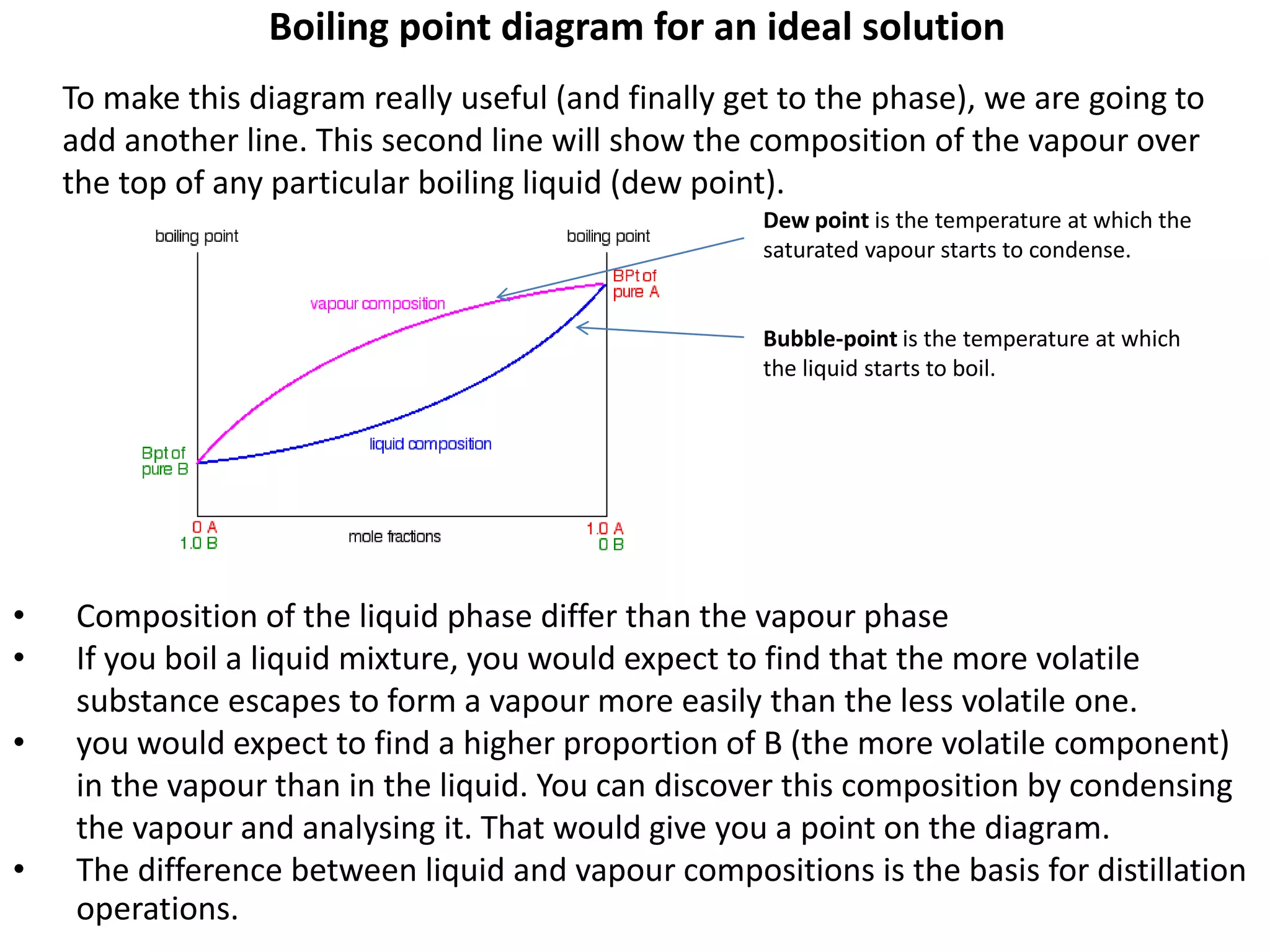
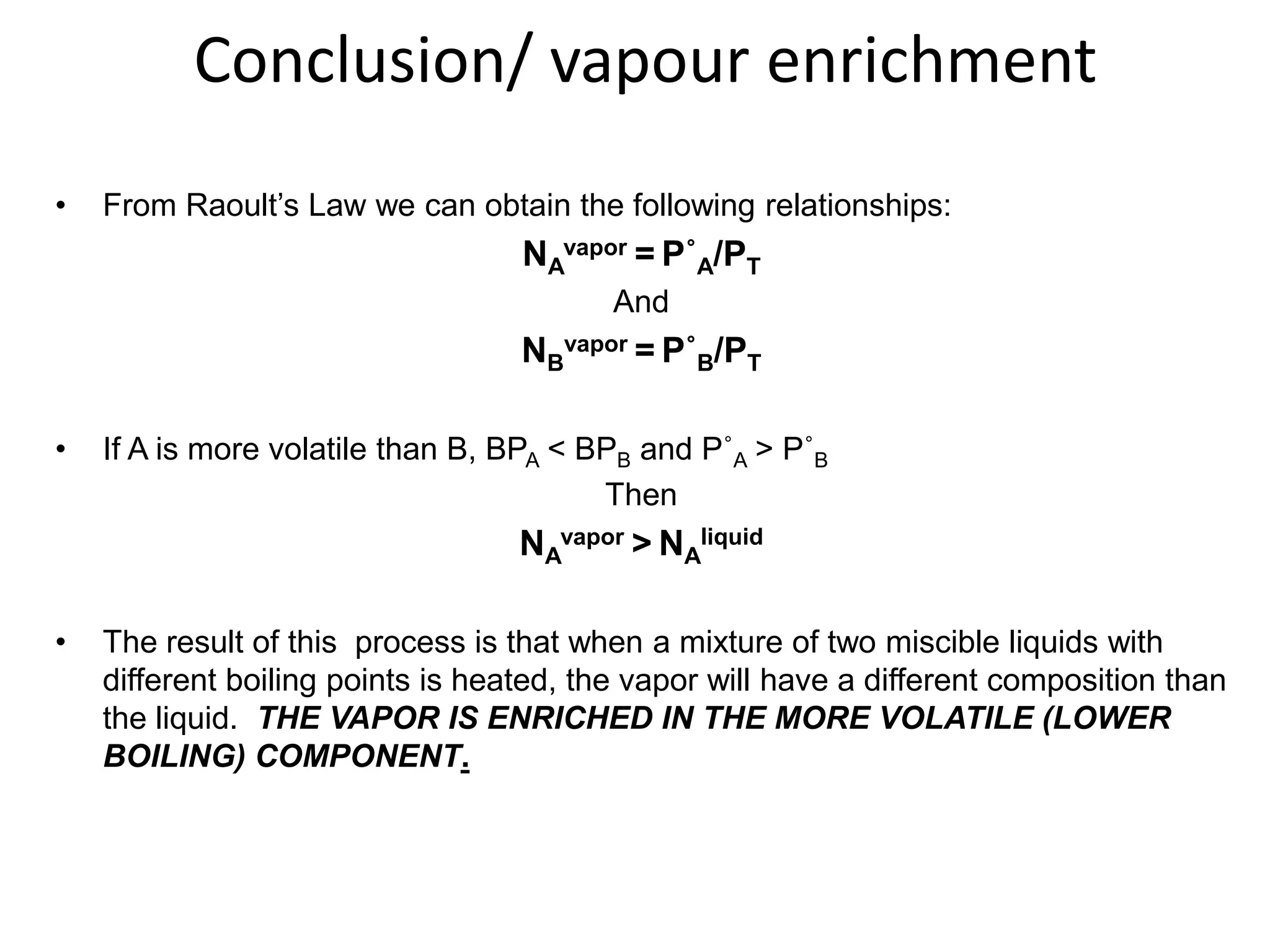
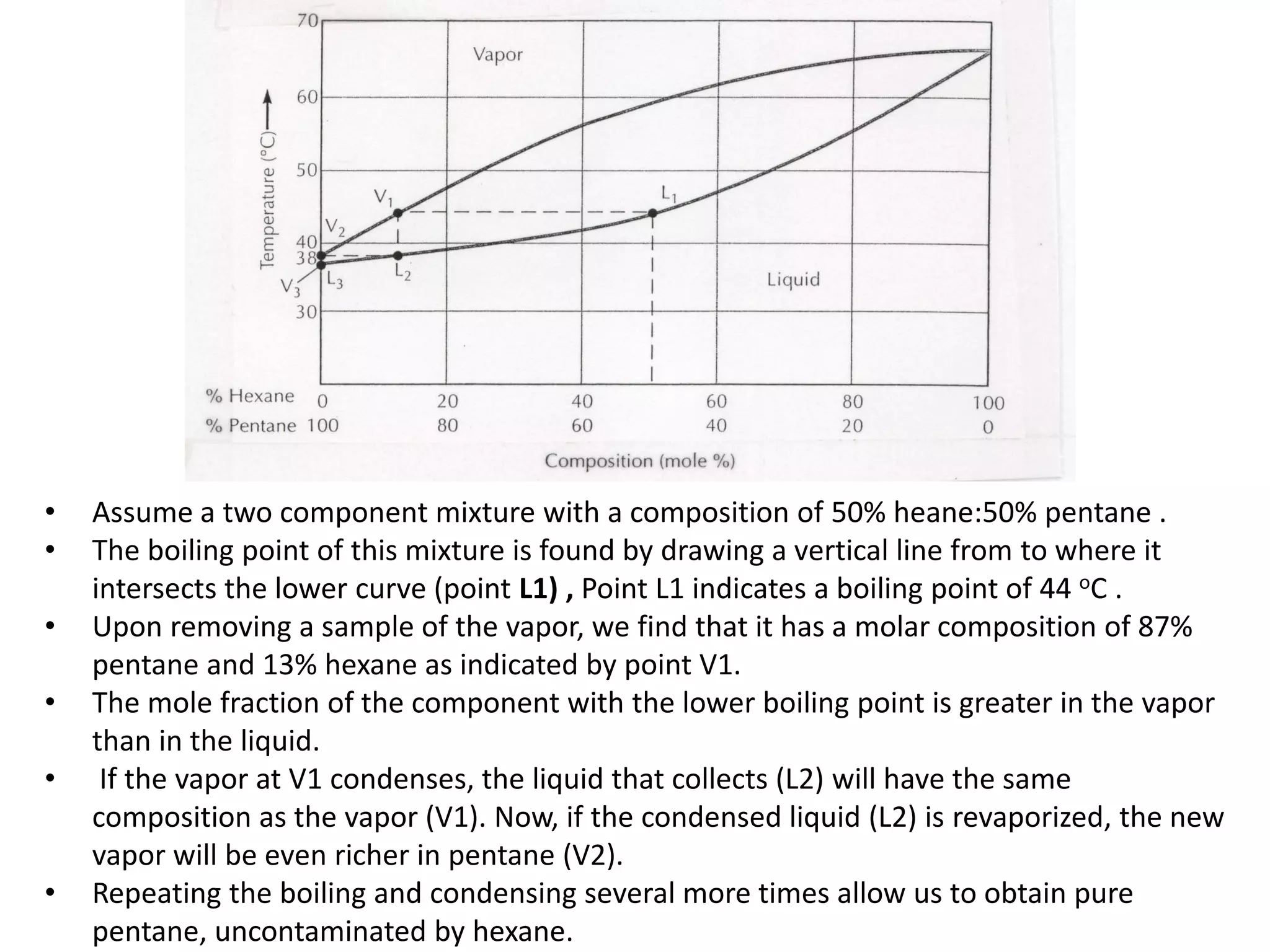
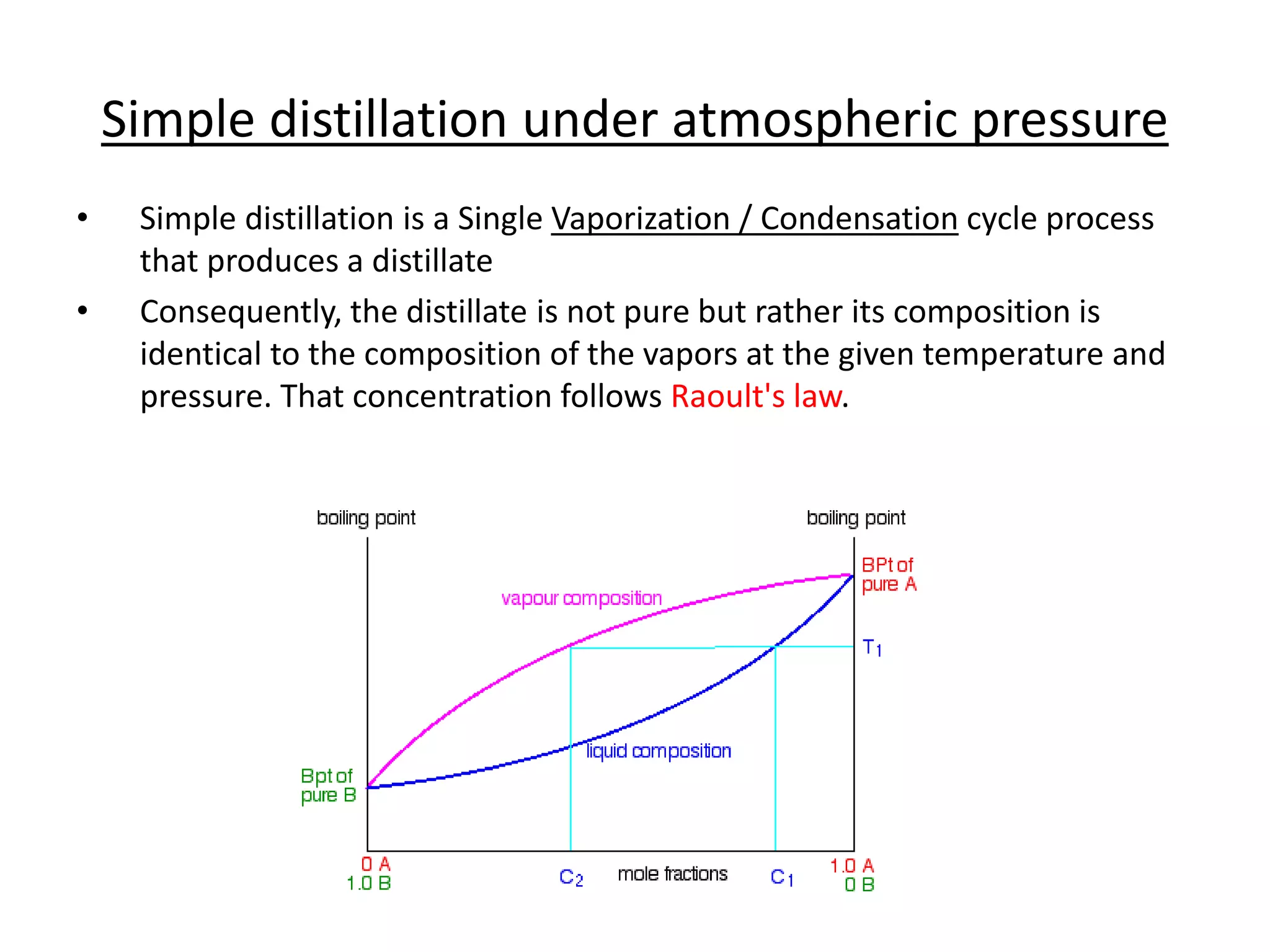
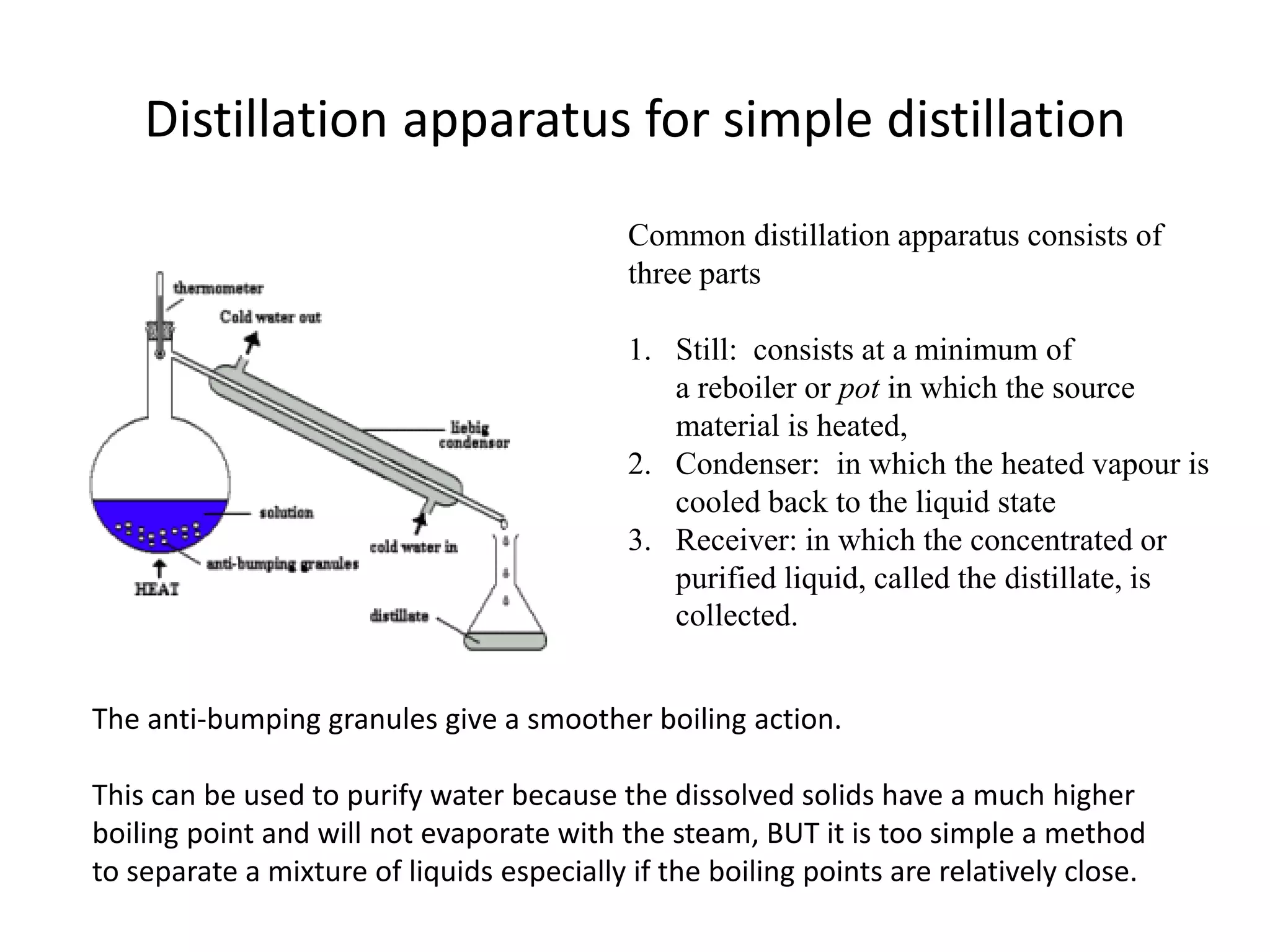
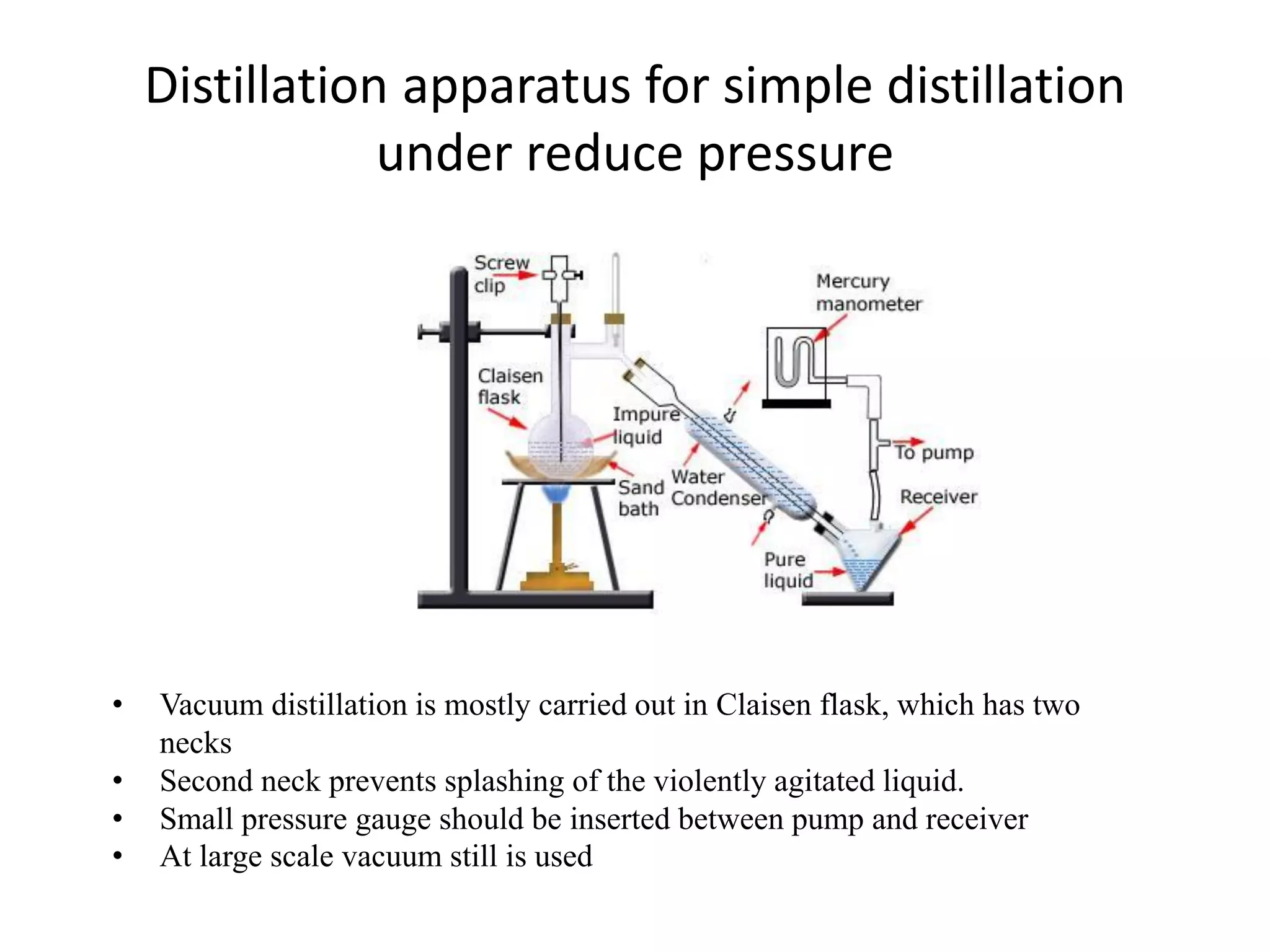
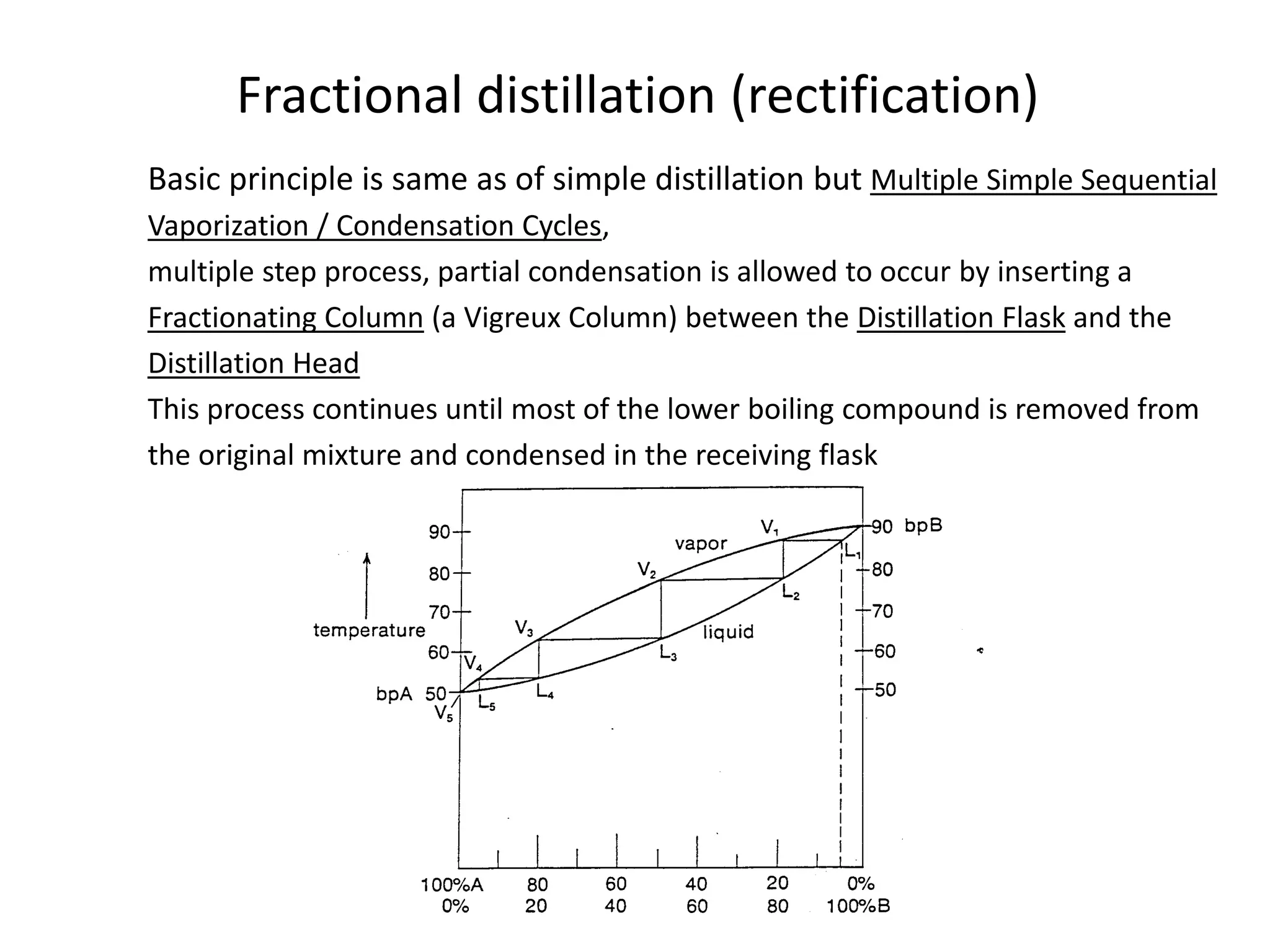
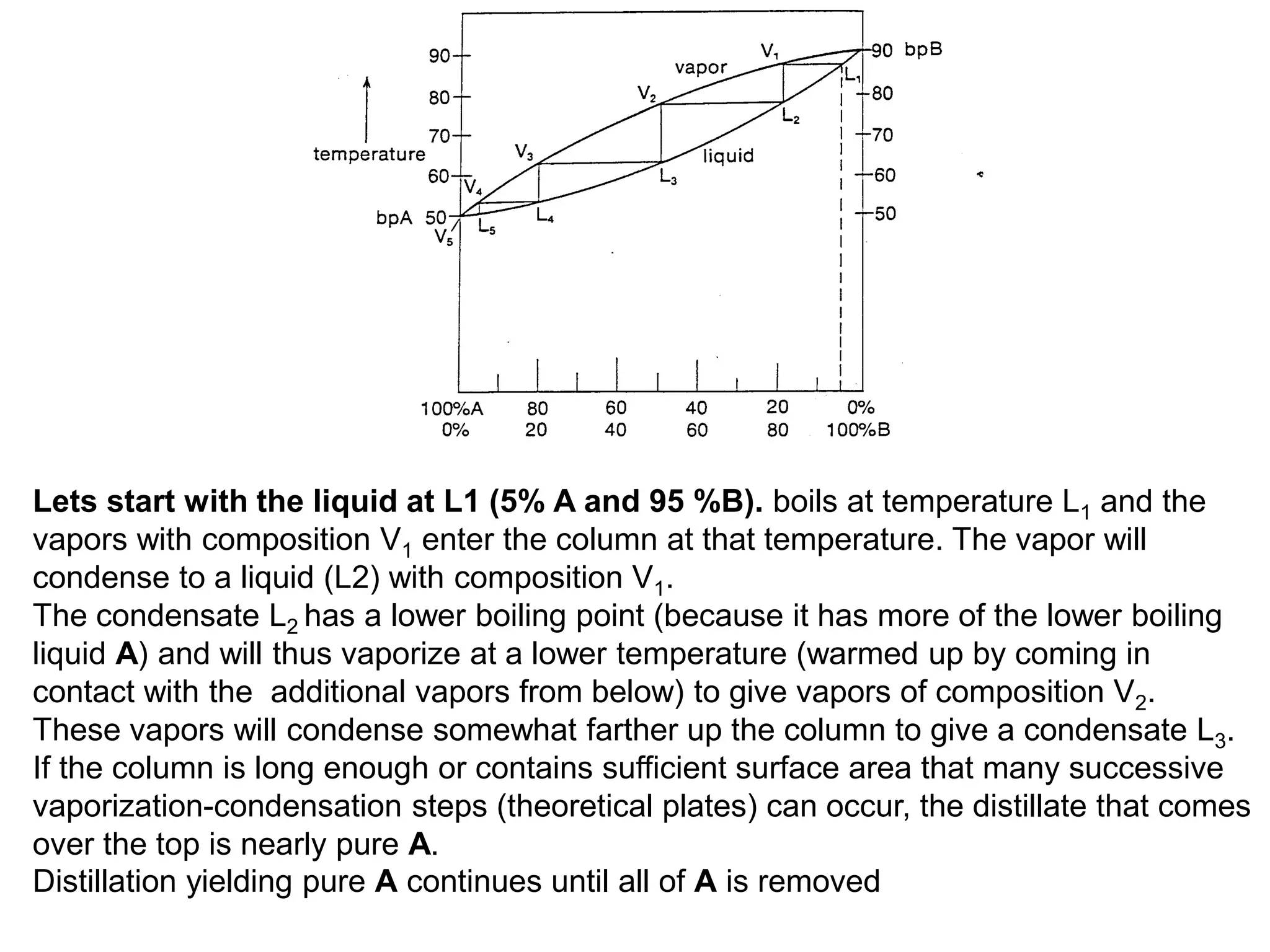
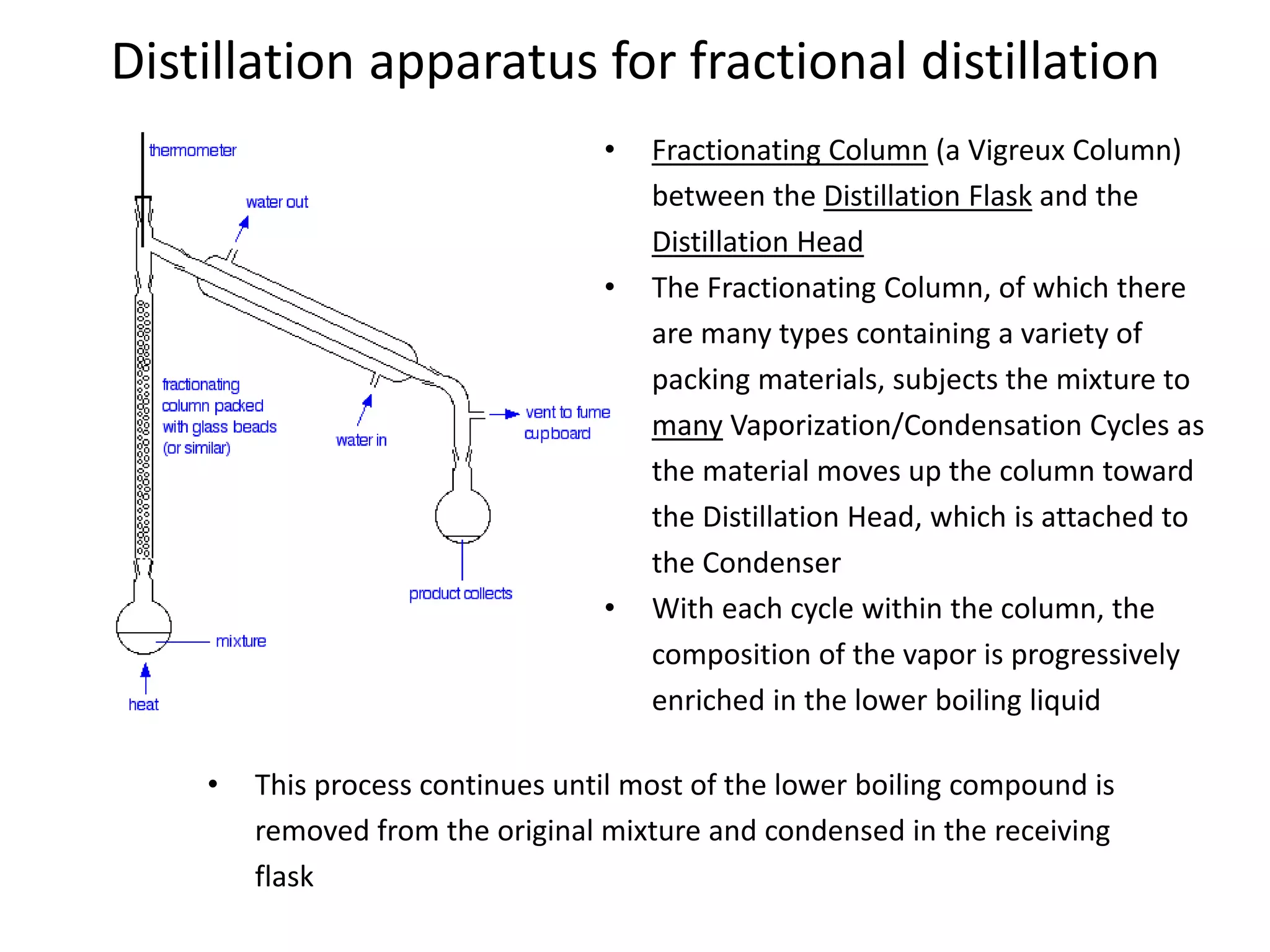
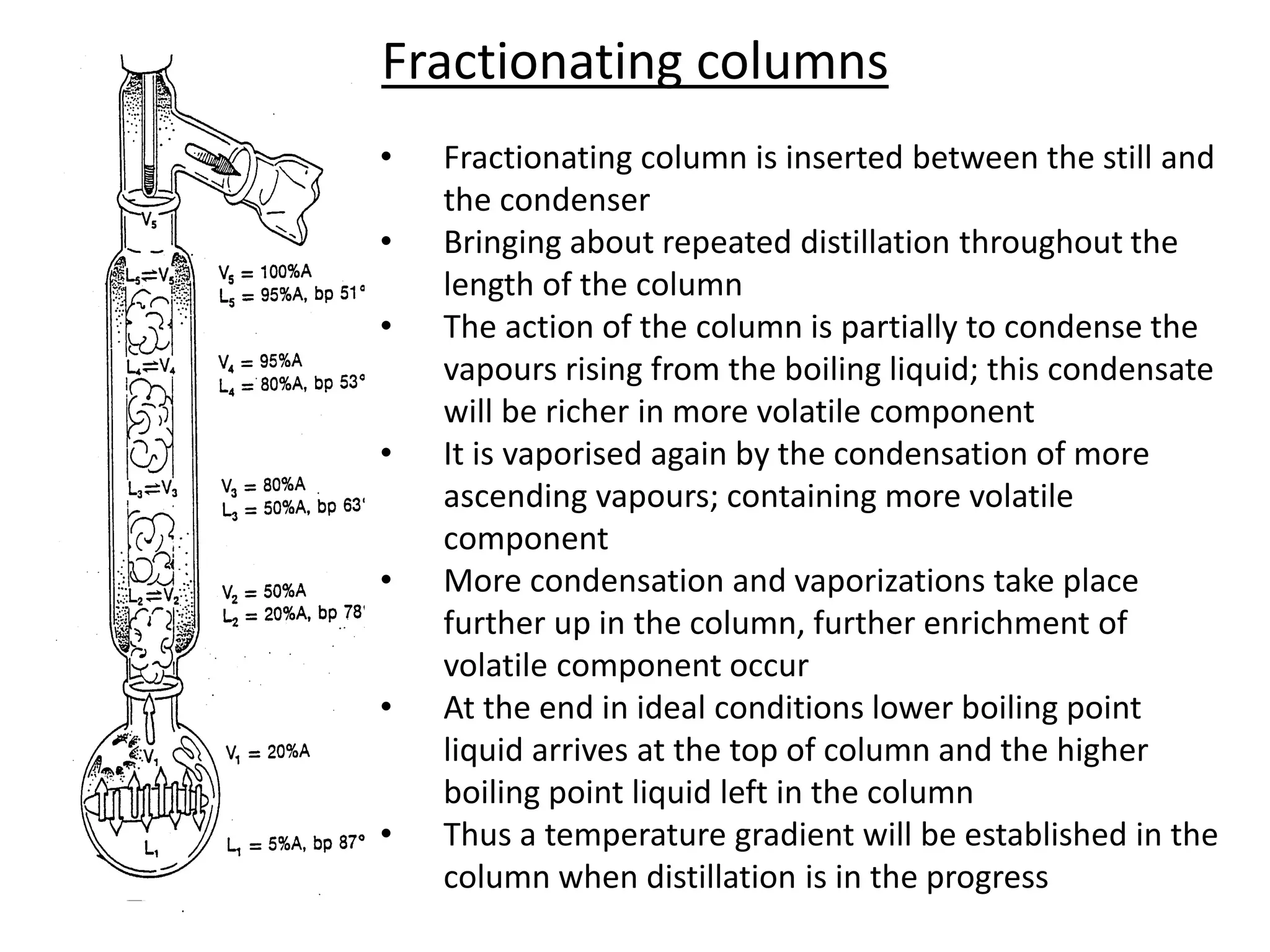
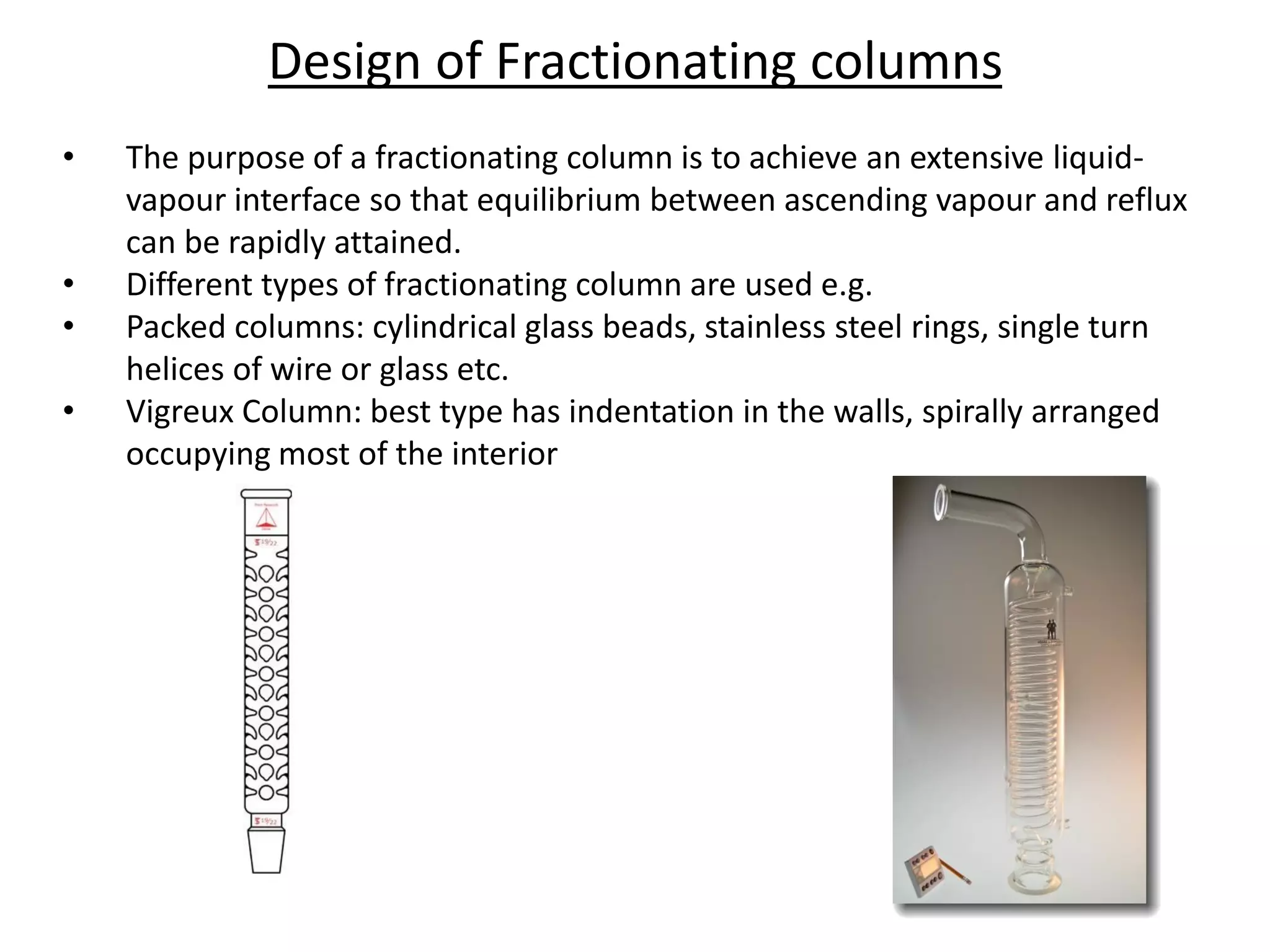
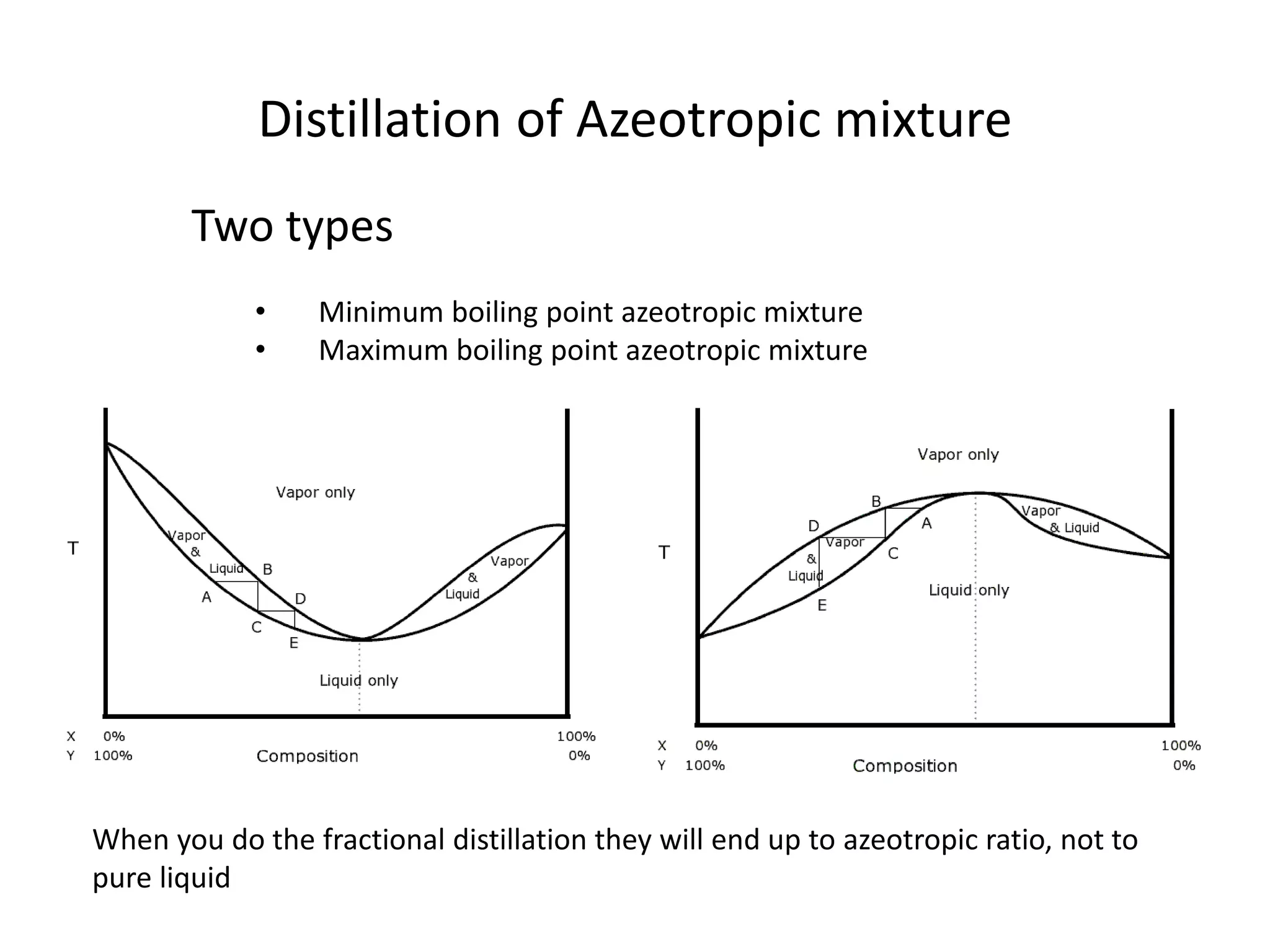
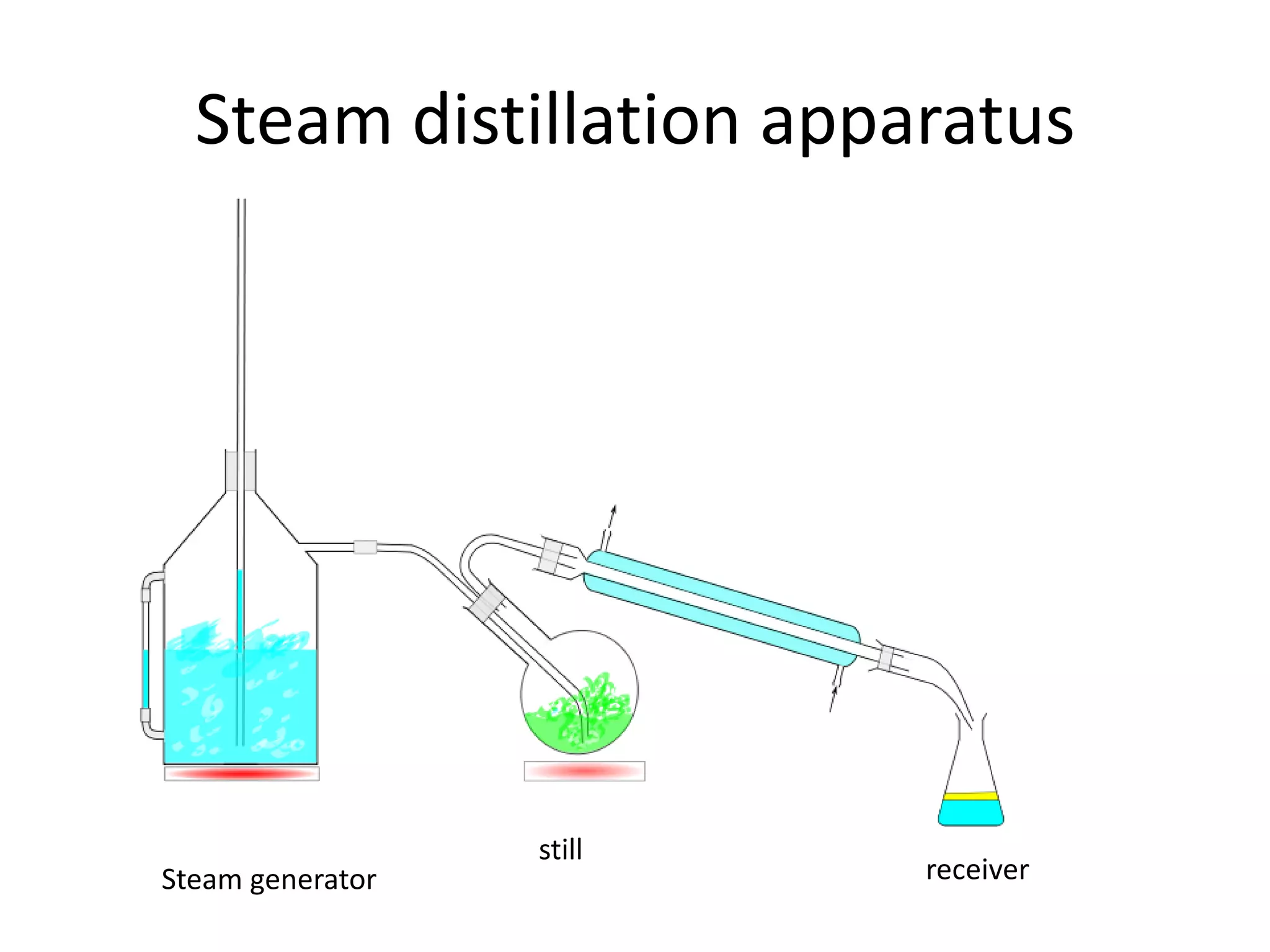
Distillation is a method of separating mixtures based on differences in volatility. It involves heating a mixture to vaporize more volatile components, which are then condensed and collected. There are several types of distillation including simple distillation, fractional distillation, steam distillation, and destructive distillation. Fractional distillation uses a fractionating column with multiple theoretical plates to achieve high purity separations, while steam distillation uses steam to reduce boiling points of heat-sensitive materials. Distillation is used in pharmacy and chemistry to extract and purify substances.