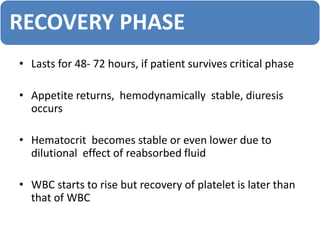
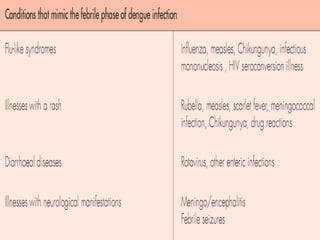
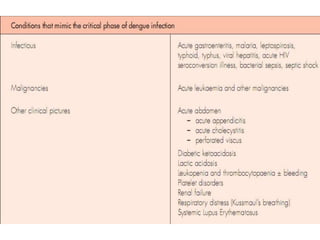
This document provides an overview of dengue, including its epidemiology, life cycle, pathogenesis, clinical features, diagnosis, management, prognosis, and prevention. Some key points:
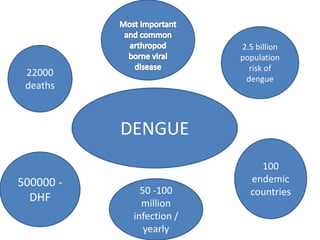
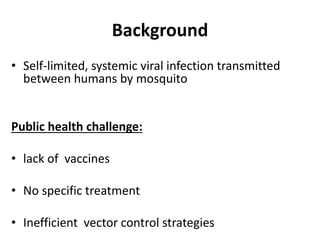
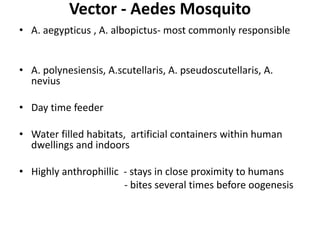
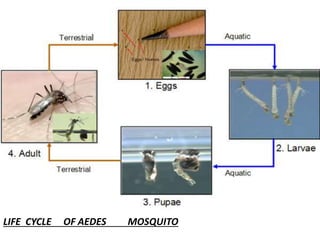
- Dengue is a self-limited viral infection transmitted by mosquitoes that infects 50-100 million people yearly and is a major public health challenge due to lack of vaccines or treatments.
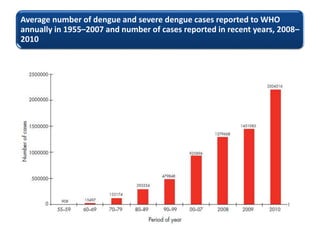
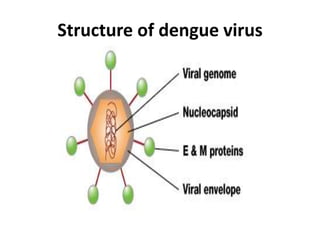
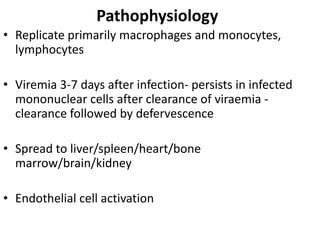
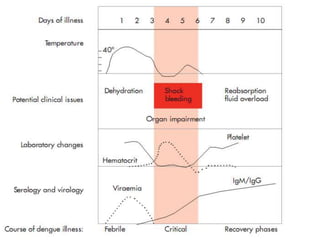
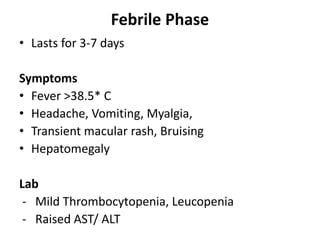
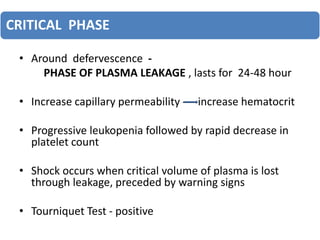
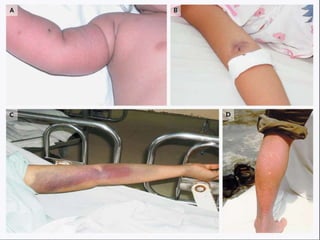
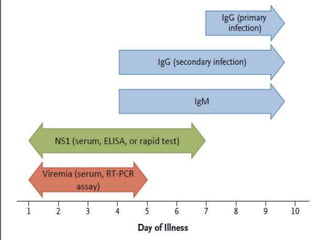
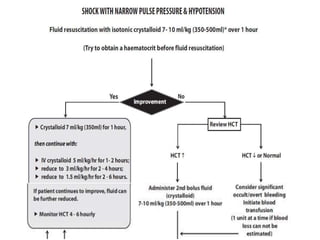
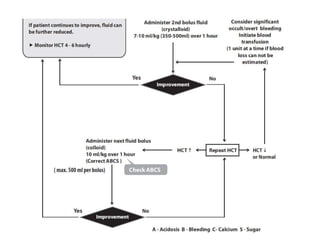
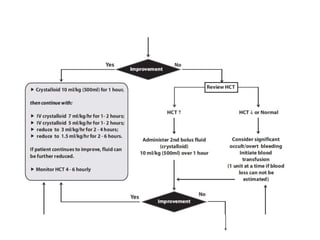
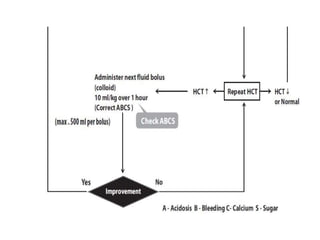
- There are four serotypes of the dengue virus. Infection causes an acute febrile illness that in some cases progresses to severe dengue with plasma leakage and potential complications including shock.
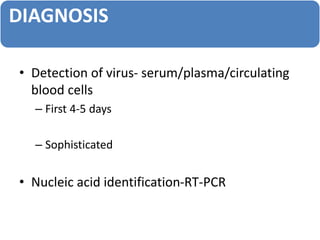
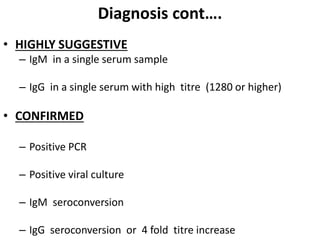
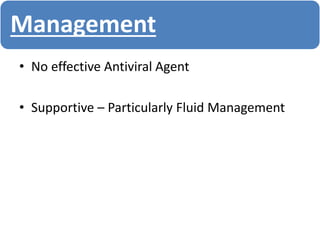
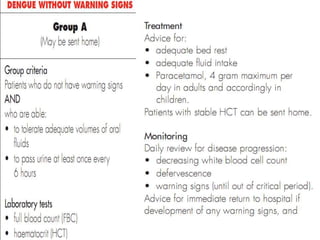
- Diagnosis is based on virus detection, serology, or PCR. Management focuses on supportive care and fluid management. Prevention emphasizes mosquito control