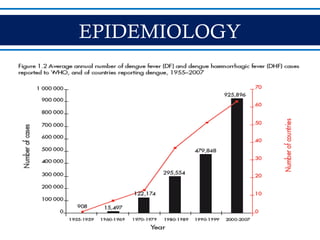
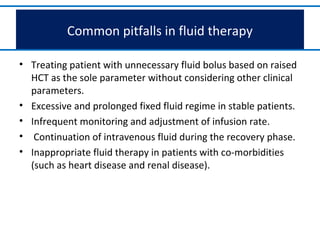
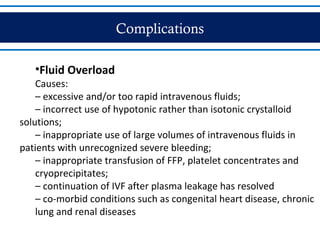
- Dengue is a mosquito-borne viral disease spread by Aedes mosquitoes, infecting 50 million people annually. It has four serotypes.
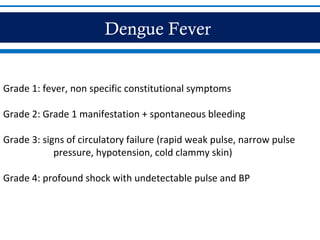
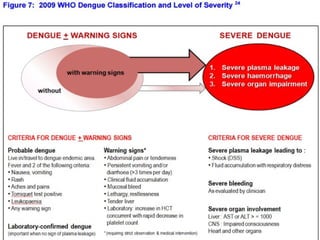
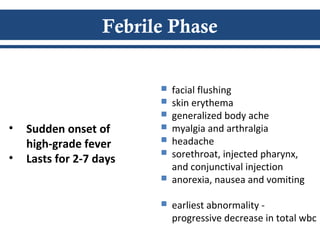
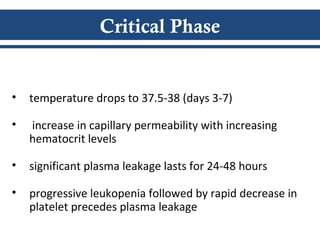
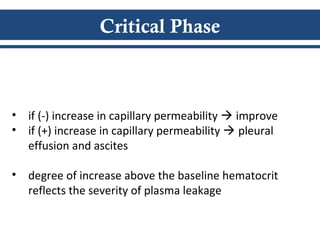
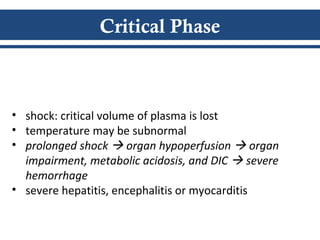
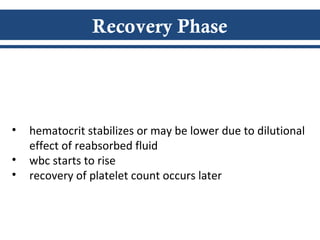
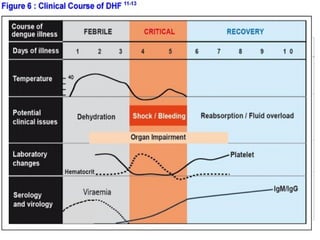
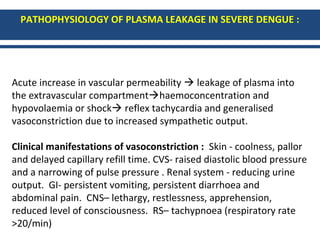
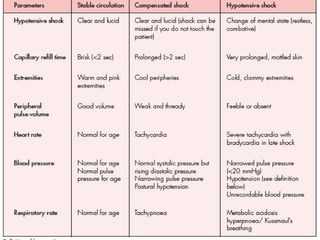
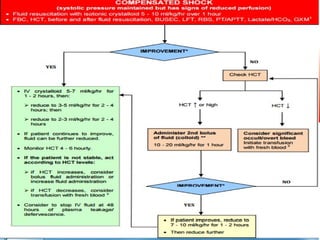
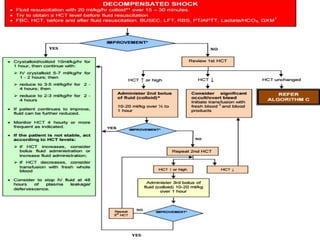
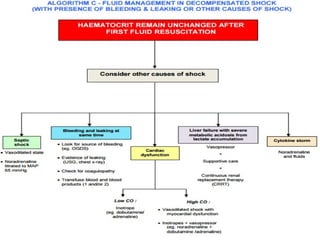
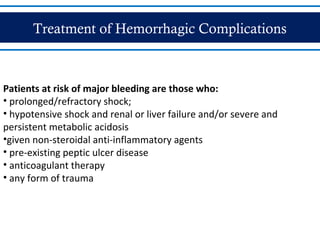
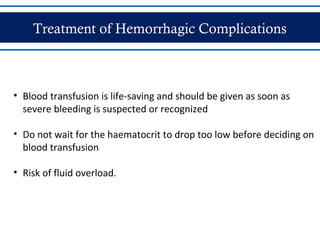
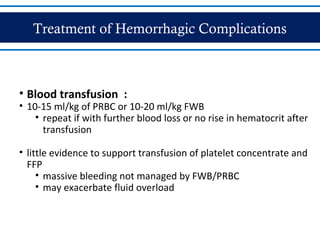
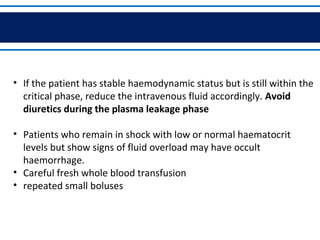
- There are three phases of dengue fever: febrile, critical, and recovery. The critical phase involves increased capillary permeability leading to plasma leakage and potential shock.
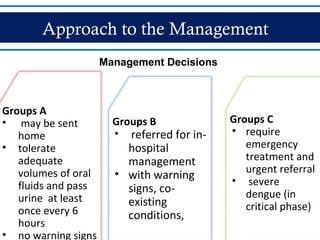
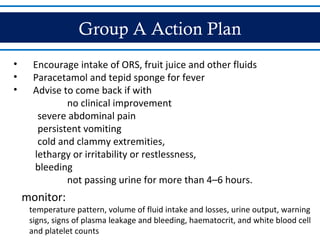
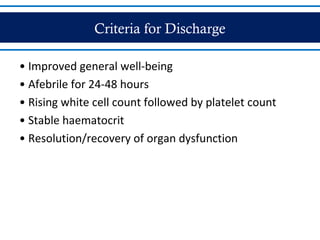
- Patients are classified into Groups A, B, or C depending on severity of symptoms. Group A can be sent home with oral rehydration. Group B requires hospitalization for intravenous fluids. Group C has severe dengue and requires emergency treatment, intensive care, and blood transfusion.