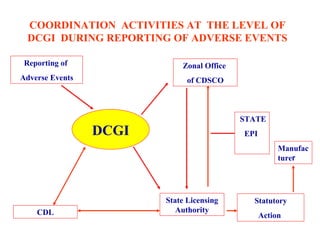
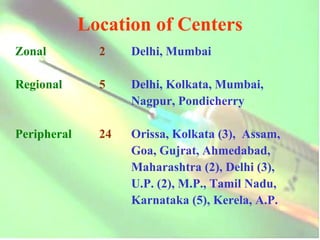
The document discusses regulatory requirements and procedures for approval of new vaccines in India. It provides definitions of key terms like drugs, new drugs and vaccines. It describes the information and data required to be submitted for approval, including safety and efficacy data from clinical trials. It also discusses post-marketing surveillance requirements and procedures for investigating and reporting adverse events following immunization.