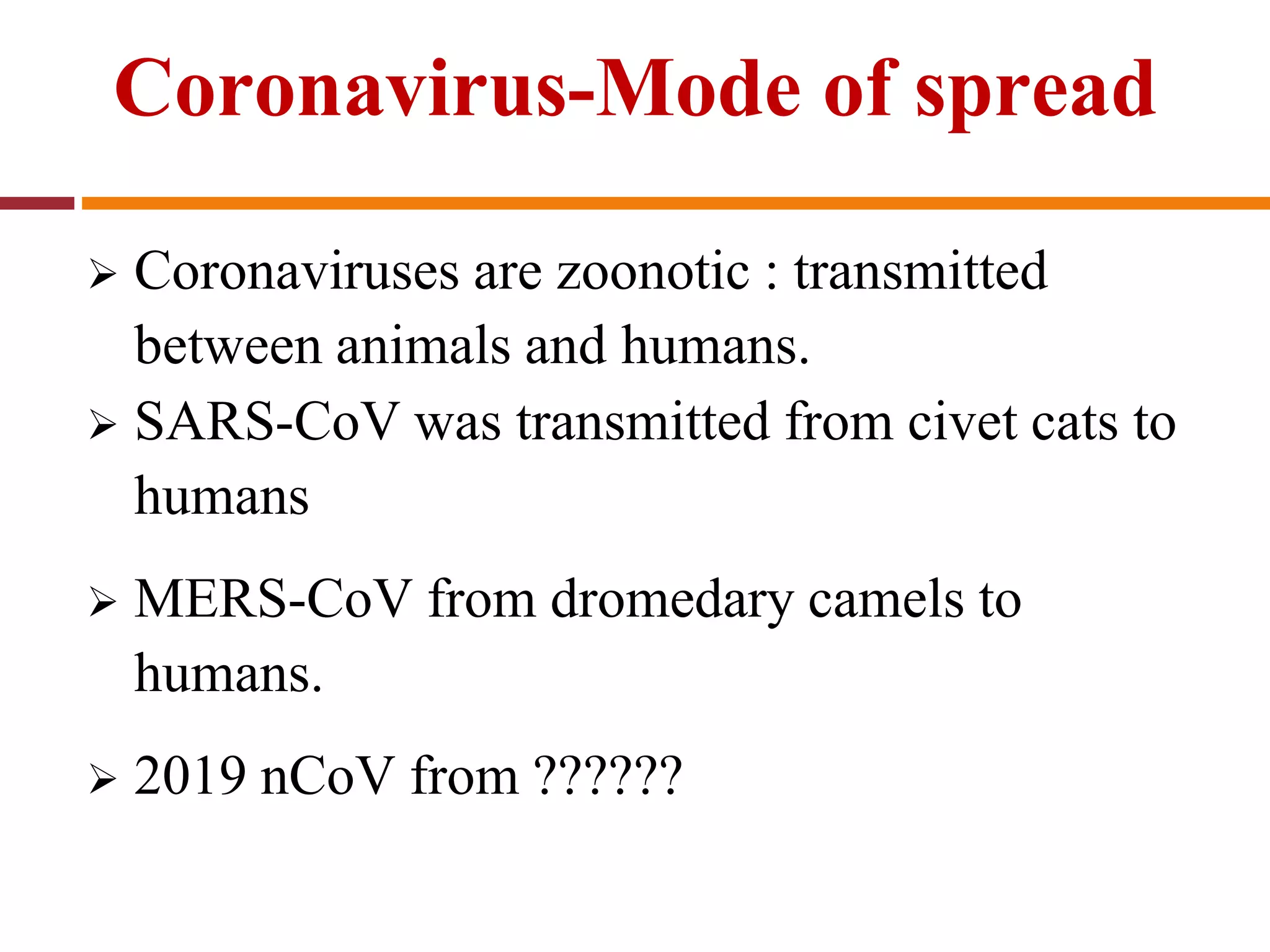
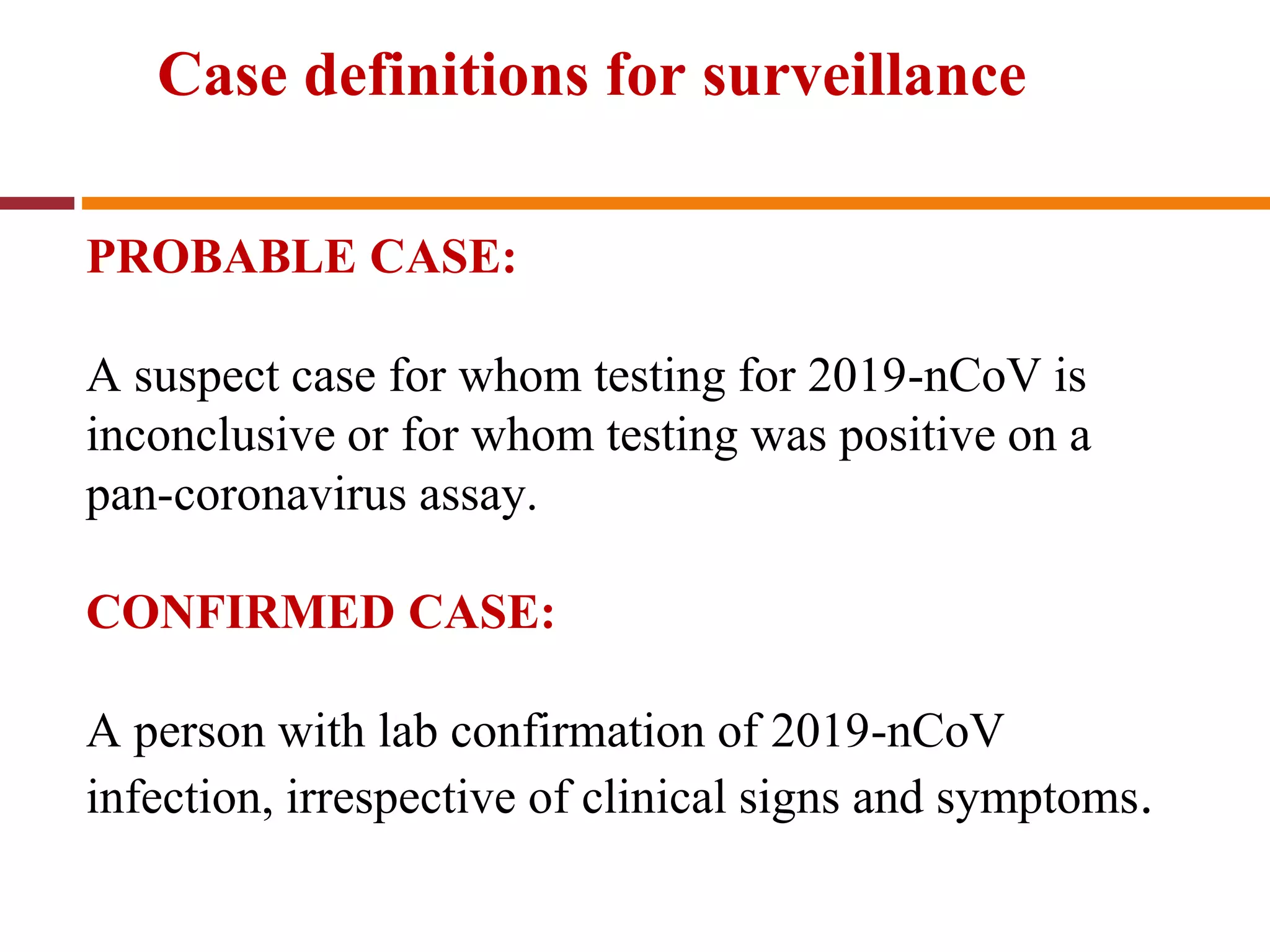
The document discusses the 2019 novel coronavirus (COVID-19) outbreak that began in Wuhan, China. It provides background on coronaviruses, describing them as enveloped RNA viruses that typically infect animals but sometimes jump to humans. The document summarizes key details about past outbreaks of MERS-CoV and SARS-CoV, as well as available information about COVID-19 such as its origin, symptoms, transmission, diagnosis, treatment and discharge criteria for patients.






















![Clinicalpresentation
Common symptoms:
URTI : Rhinorrhoea,Sneezing, or Sore Throat
Fever [83%]
Cough [82%]
Myalgia or Fatigue [11%]
Dyspnoea[31%]
Headache[8%]
Haemoptysis
Diarrhoea
Vomiting](https://image.slidesharecdn.com/covid19-200215123356/75/Covid-19-28-2048.jpg)











