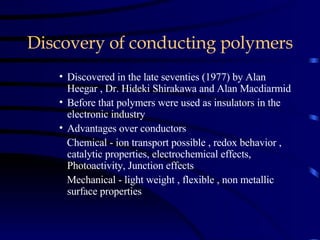
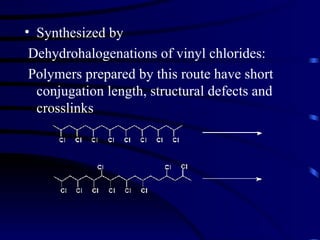
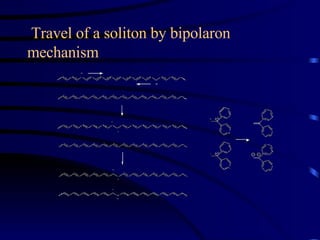
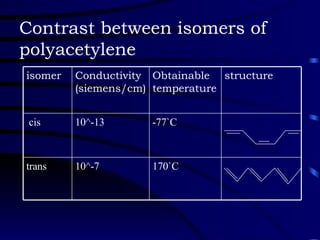
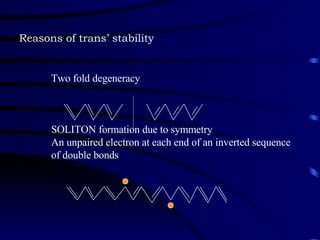
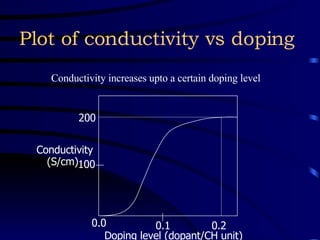
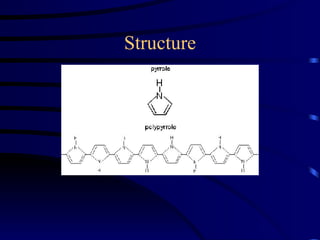
Conducting polymers were discovered in the late 1970s and represent an important class of organic polymers that can conduct electricity. They become conductive through a process called doping, where their polymer chains take on charges. This allows for charge carriers called polarons and bipolarons to form, making the material conductive. Conducting polymers have advantages over traditional conductors including light weight, flexibility, and potential applications in areas like sensors, batteries, and displays. However, issues remain around their reproducibility, stability, processing difficulties, and high costs.