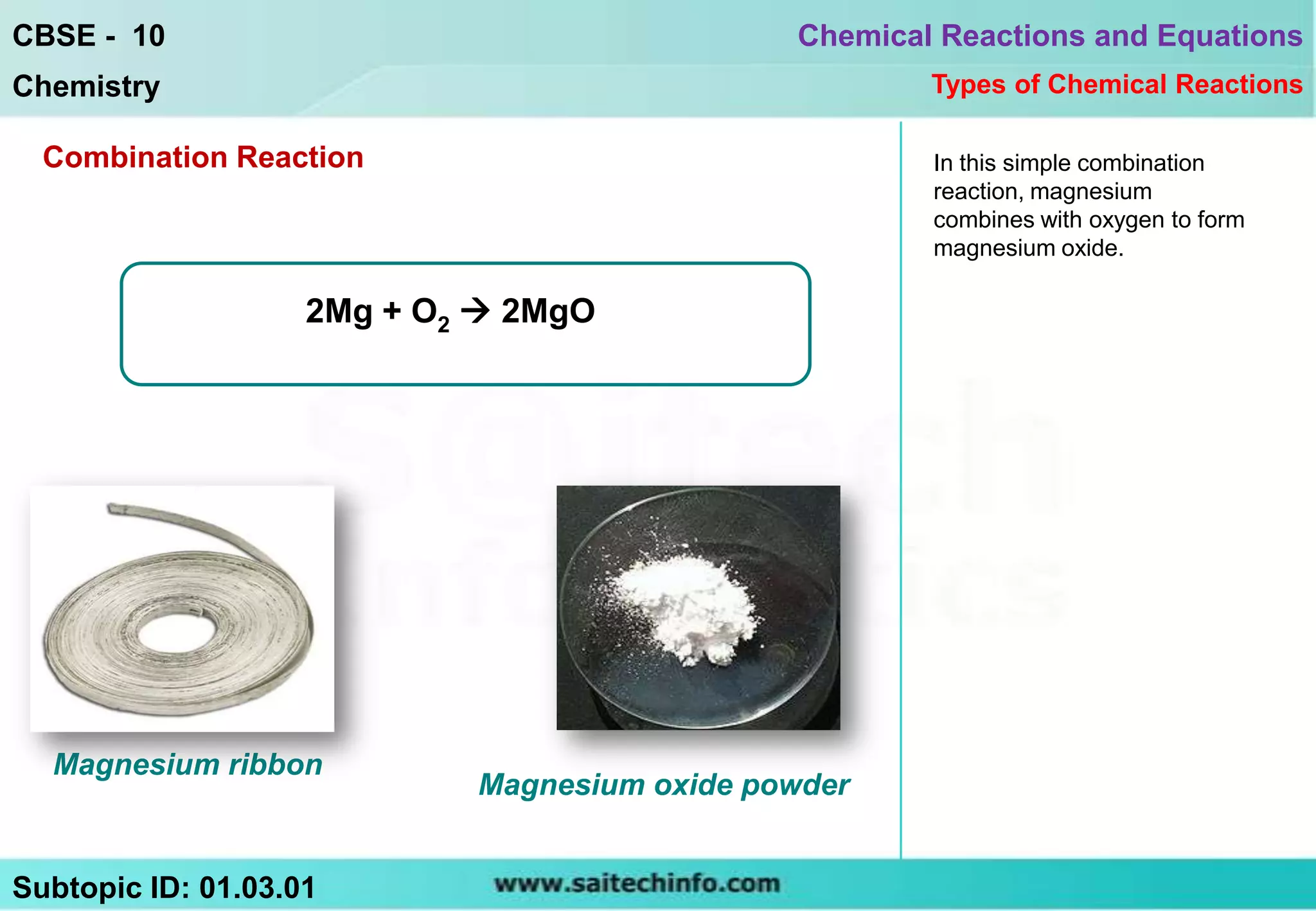
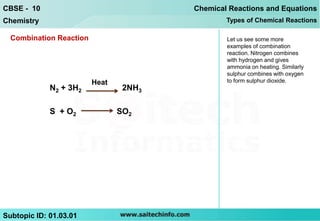
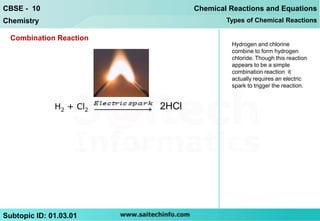
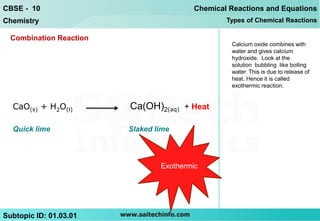
The document discusses combination reactions, which are chemical reactions where two or more substances combine to form a single new substance. It provides several examples of combination reactions, such as magnesium and oxygen combining to form magnesium oxide, sodium and chlorine combining to form sodium chloride when heated, and calcium oxide combining with water to form calcium hydroxide and release heat in an exothermic reaction. The document notes that combination reactions can be exothermic, as seen in the example of calcium oxide and water, and lists other examples of exothermic reactions like respiration and burning natural gas.