Embed presentation
Downloaded 91 times
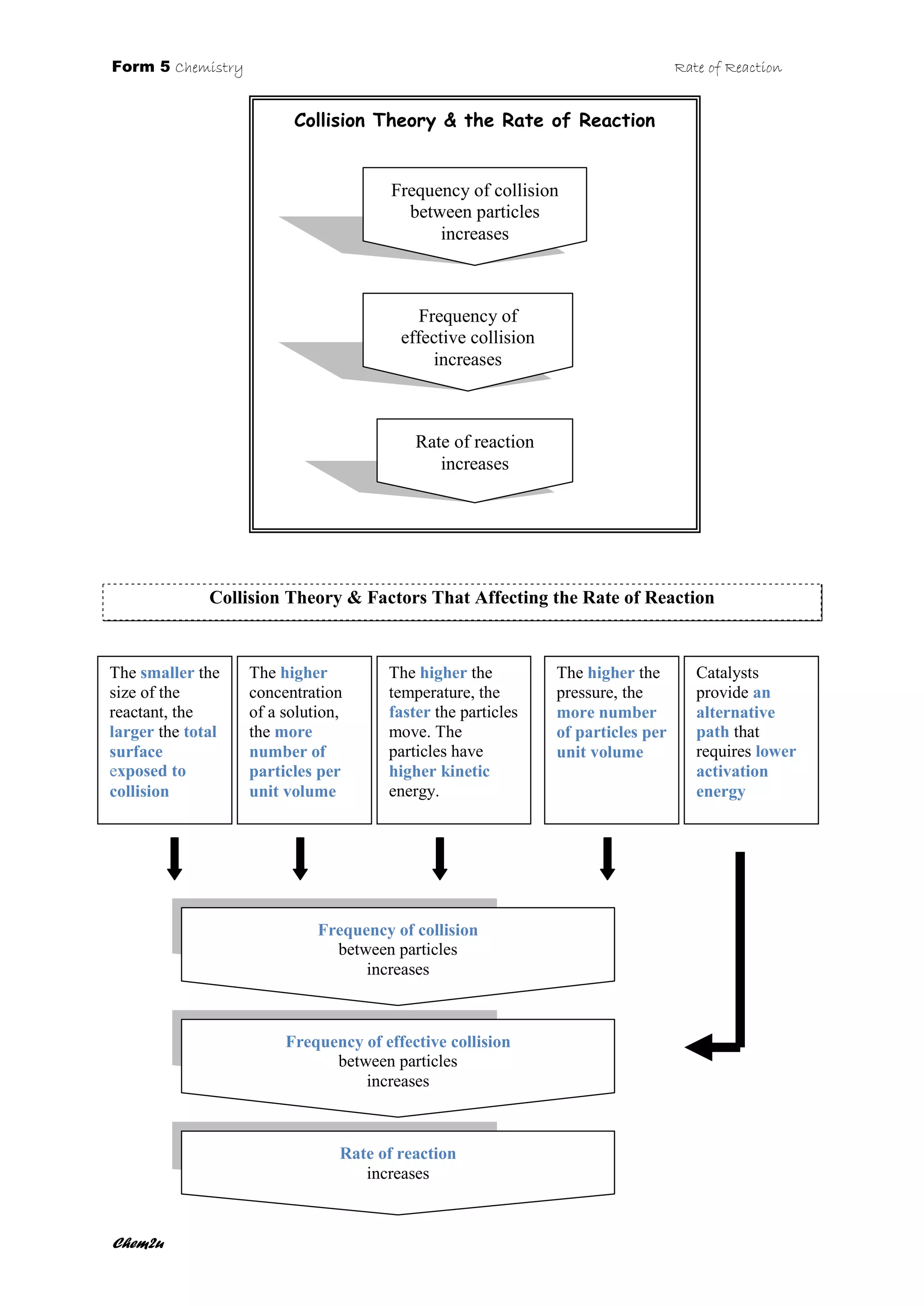
The document discusses collision theory and the factors that affect the rate of reaction. It states that as the frequency of collisions between particles increases, so too does the frequency of effective collisions, leading to an increased rate of reaction. Several factors are described that can increase the collision frequency, such as decreasing particle size, increasing concentration, raising temperature, or increasing pressure. Catalysts also provide an alternative reaction pathway requiring less activation energy.
