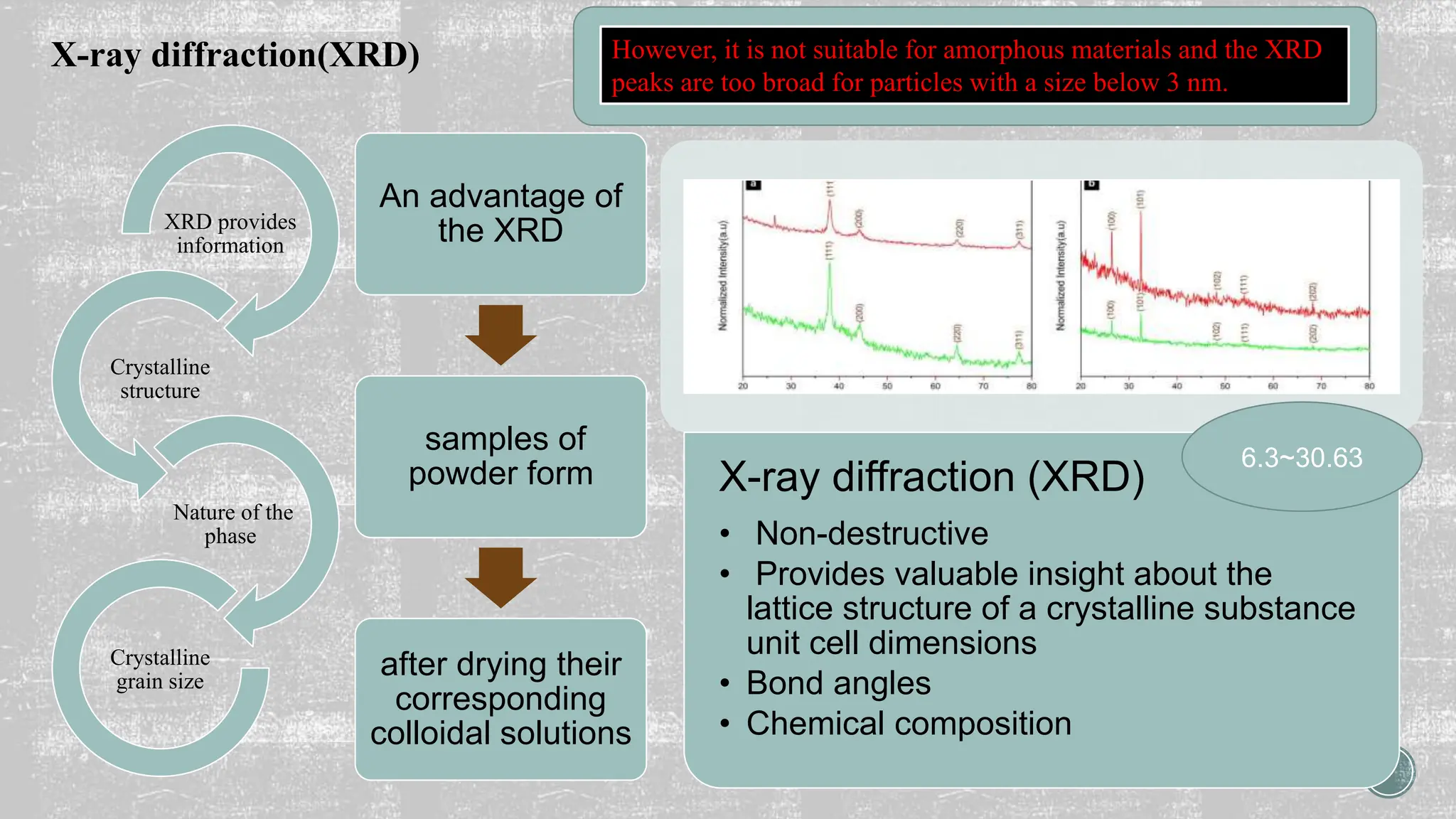
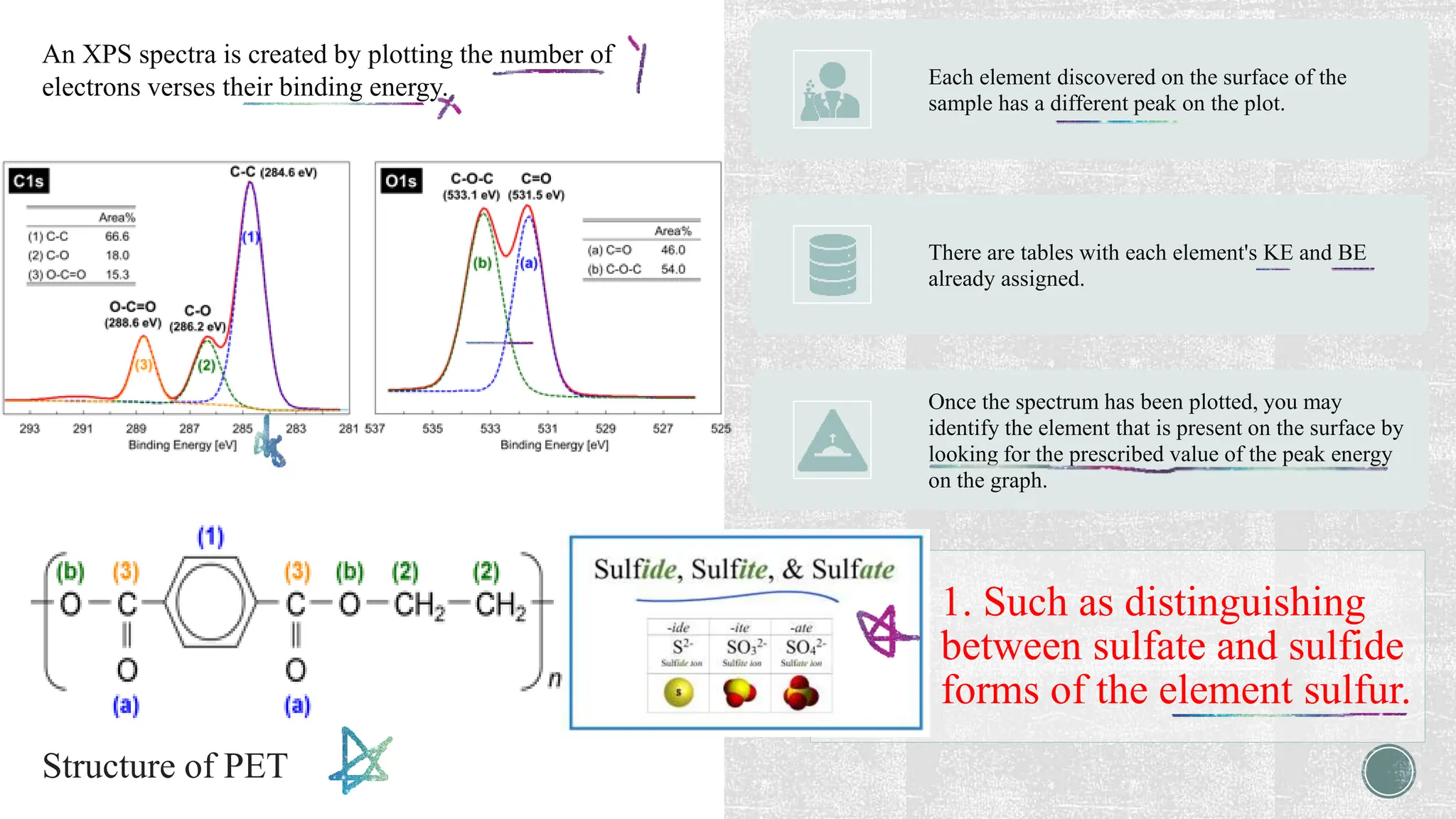
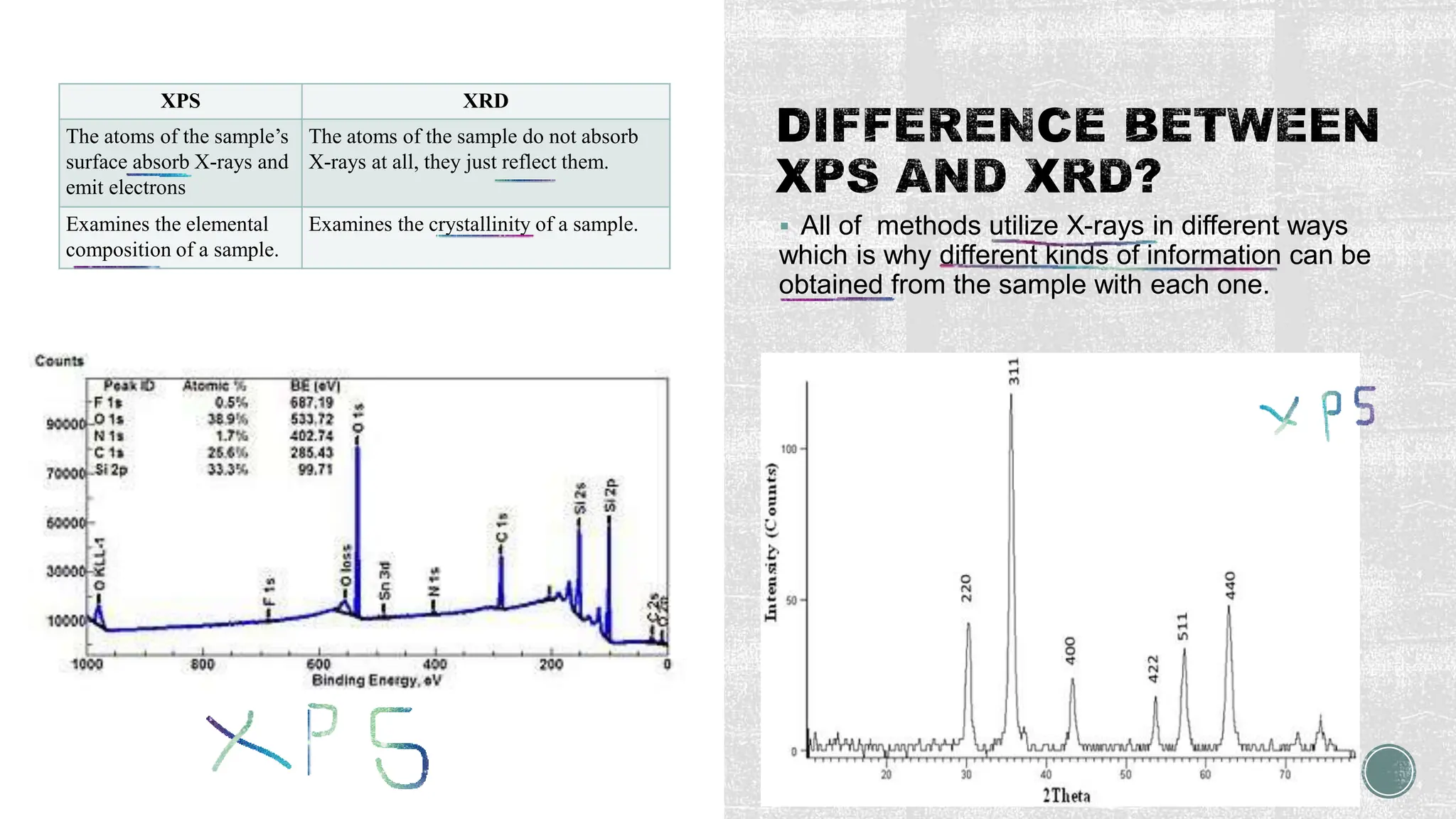
The document discusses the importance of characterizing nanomaterials using various techniques to understand their physical properties, including size and elemental composition. Key methods highlighted include scanning electron microscopy (SEM), x-ray diffraction (XRD), and energy dispersive x-ray (EDX), each providing unique insights into material characteristics. Additionally, x-ray photoelectron spectroscopy (XPS) is emphasized for its ability to analyze surface chemistry at an atomic level.