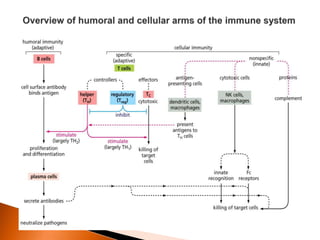
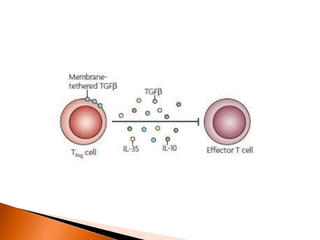
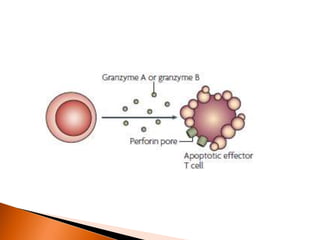
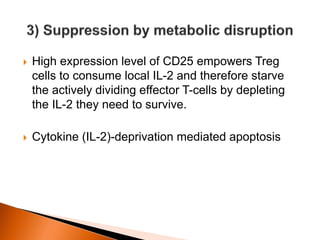
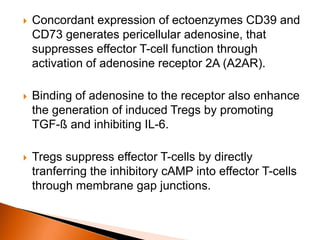
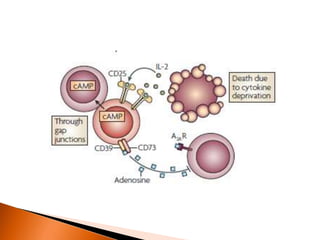
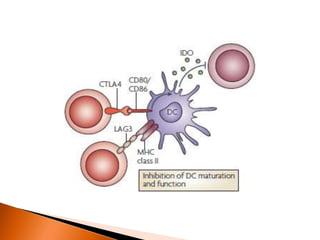
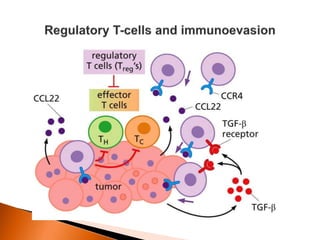
Regulatory T-cells (Tregs) help maintain self-tolerance and prevent autoimmunity by suppressing immune responses. They express FOXP3 and CD25 and function through various mechanisms like secreting inhibitory cytokines or metabolizing IL-2. Tregs are implicated in tumor immune escape by suppressing anti-tumor immunity. While Tregs are normally beneficial, in cancer high levels associate with poor prognosis by hindering immune response. Emerging immunotherapies aim to deplete or modulate Tregs to enhance anti-tumor immunity.