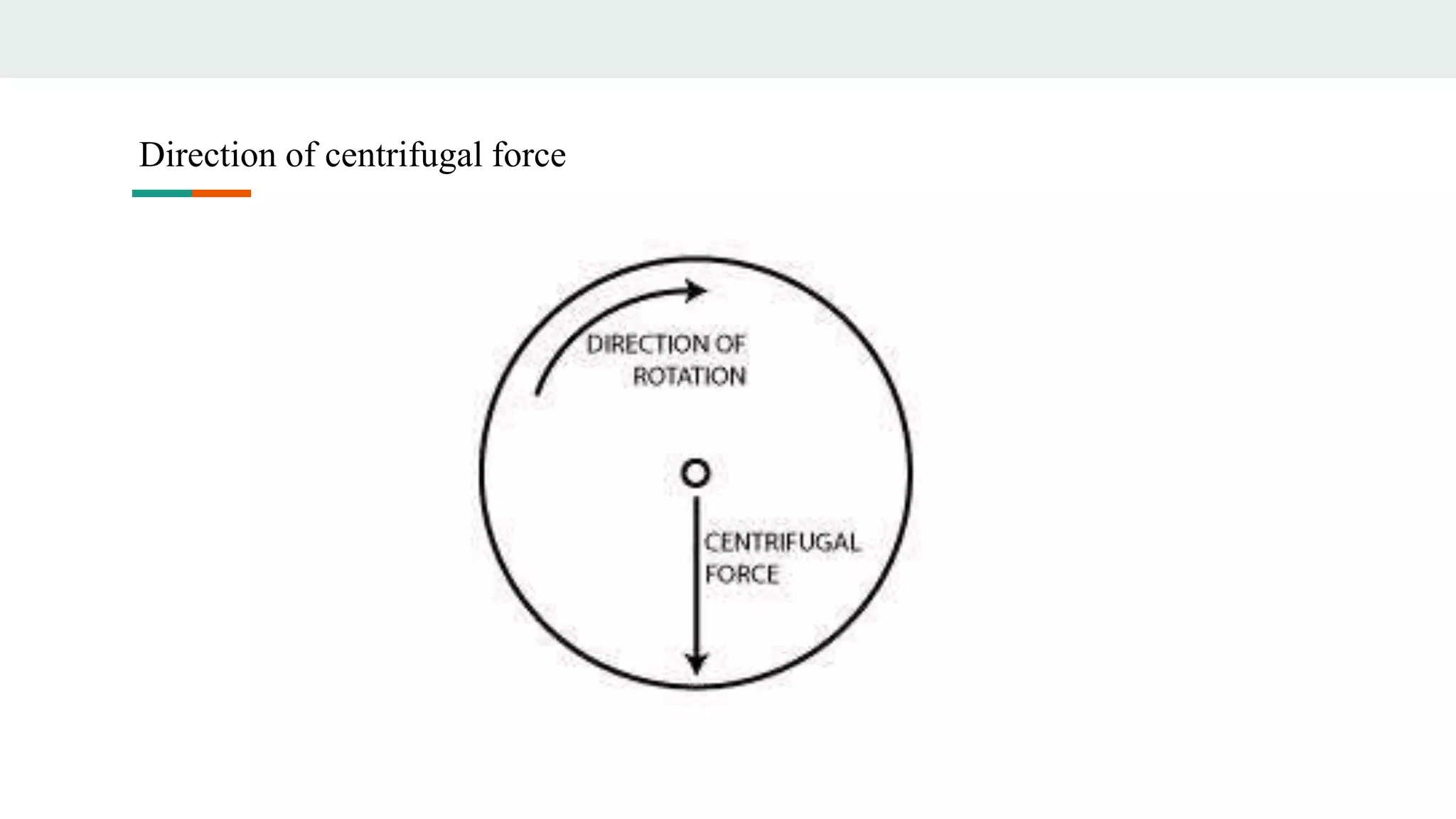
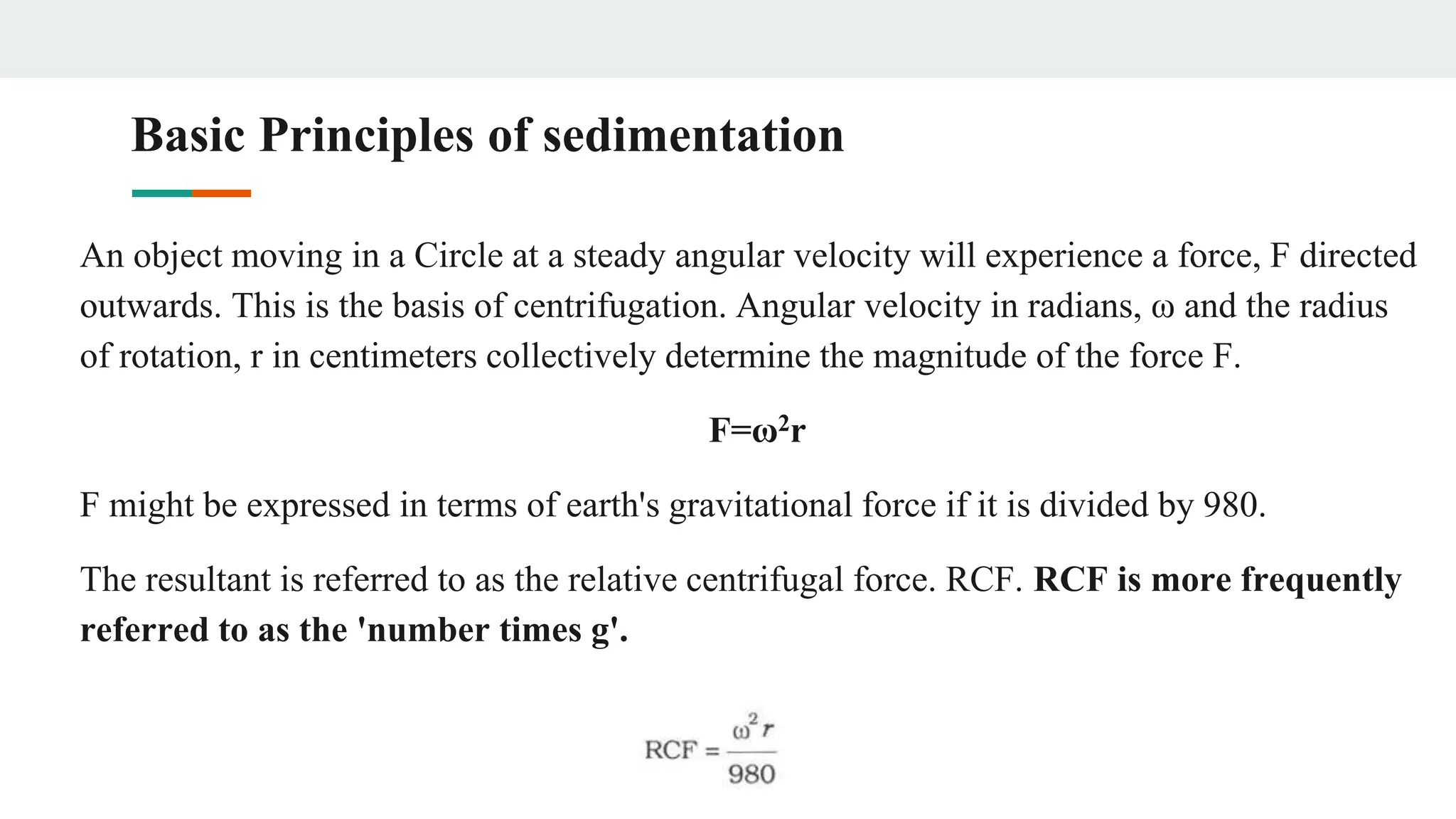
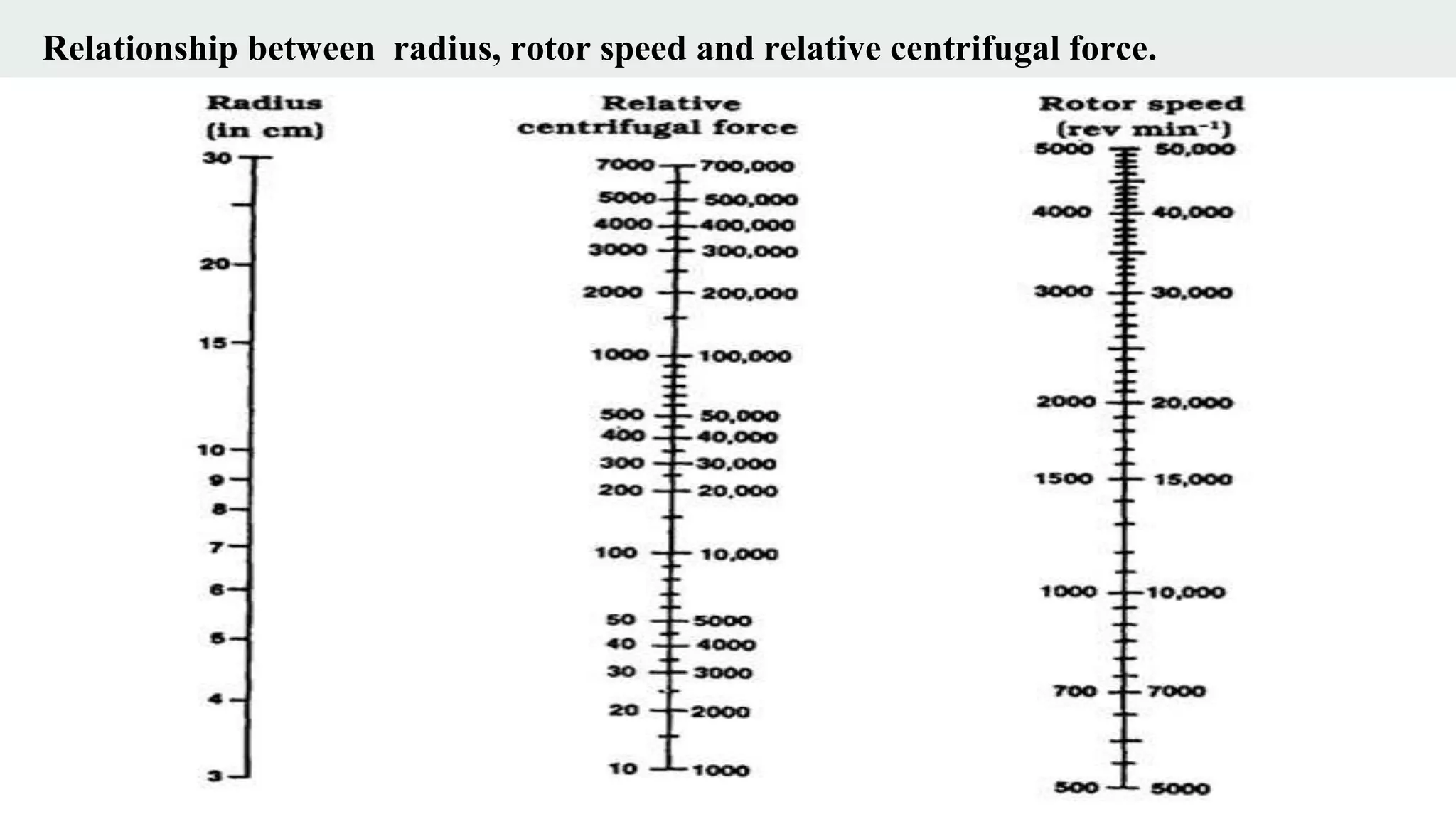
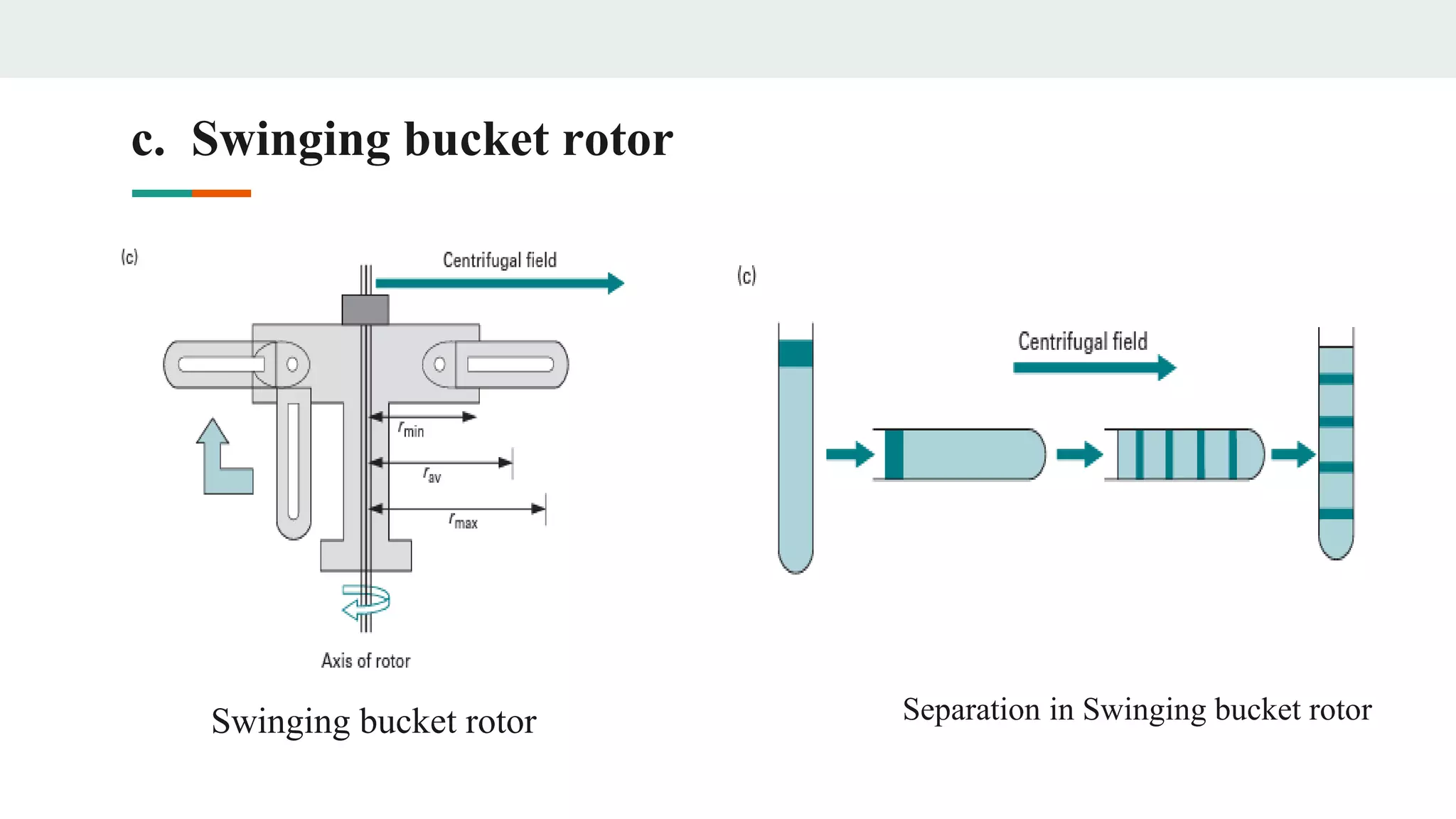
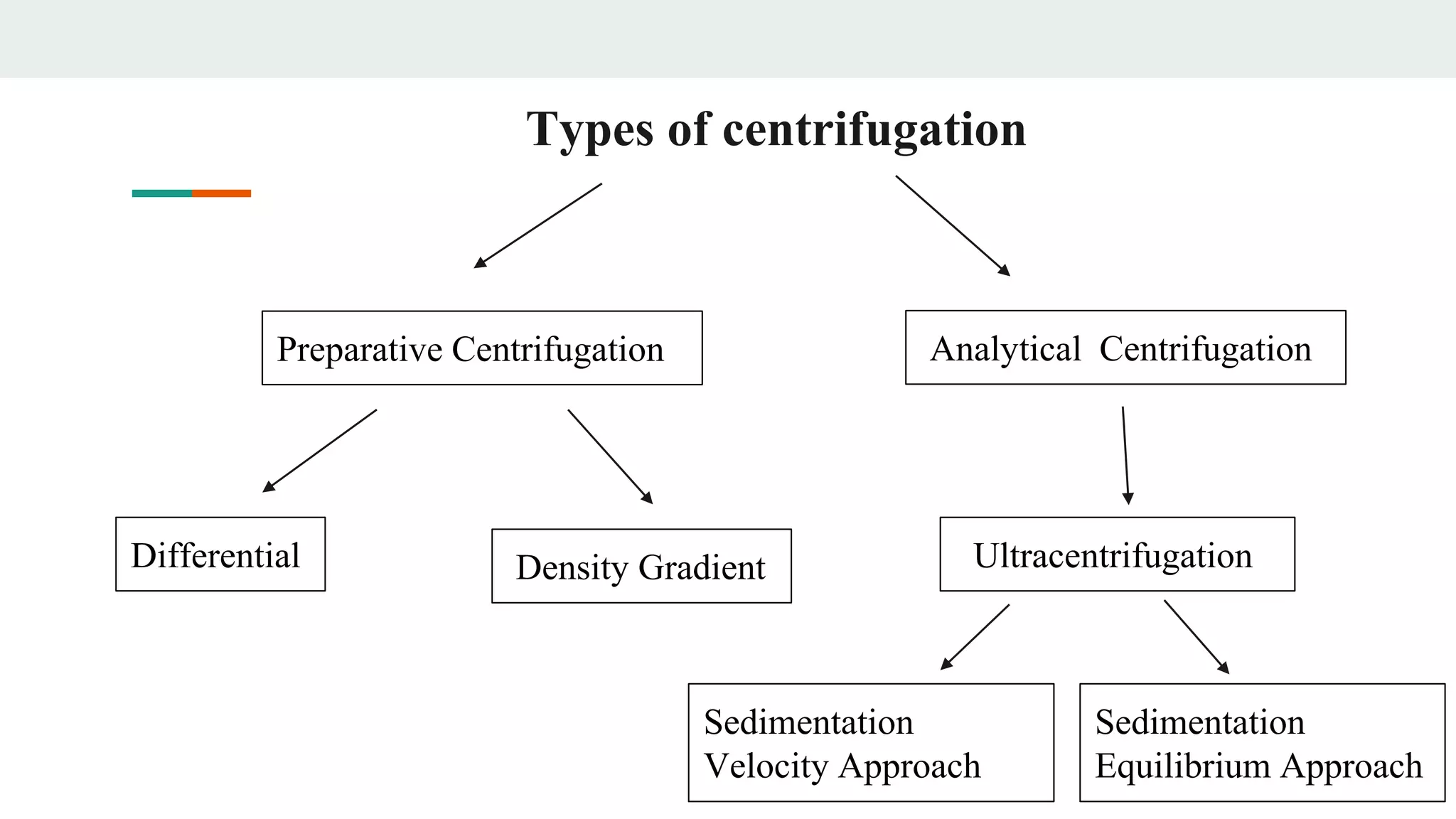
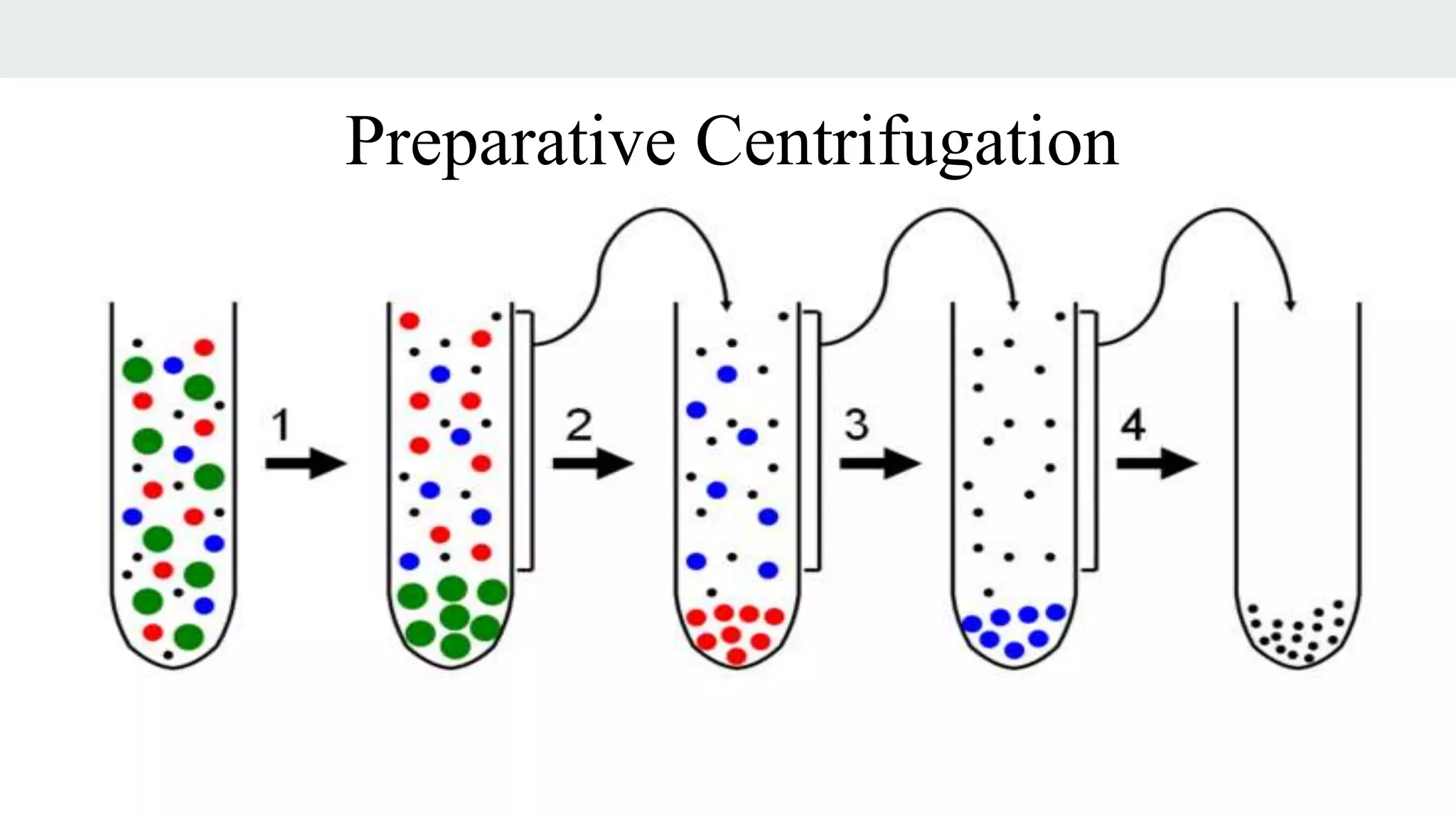
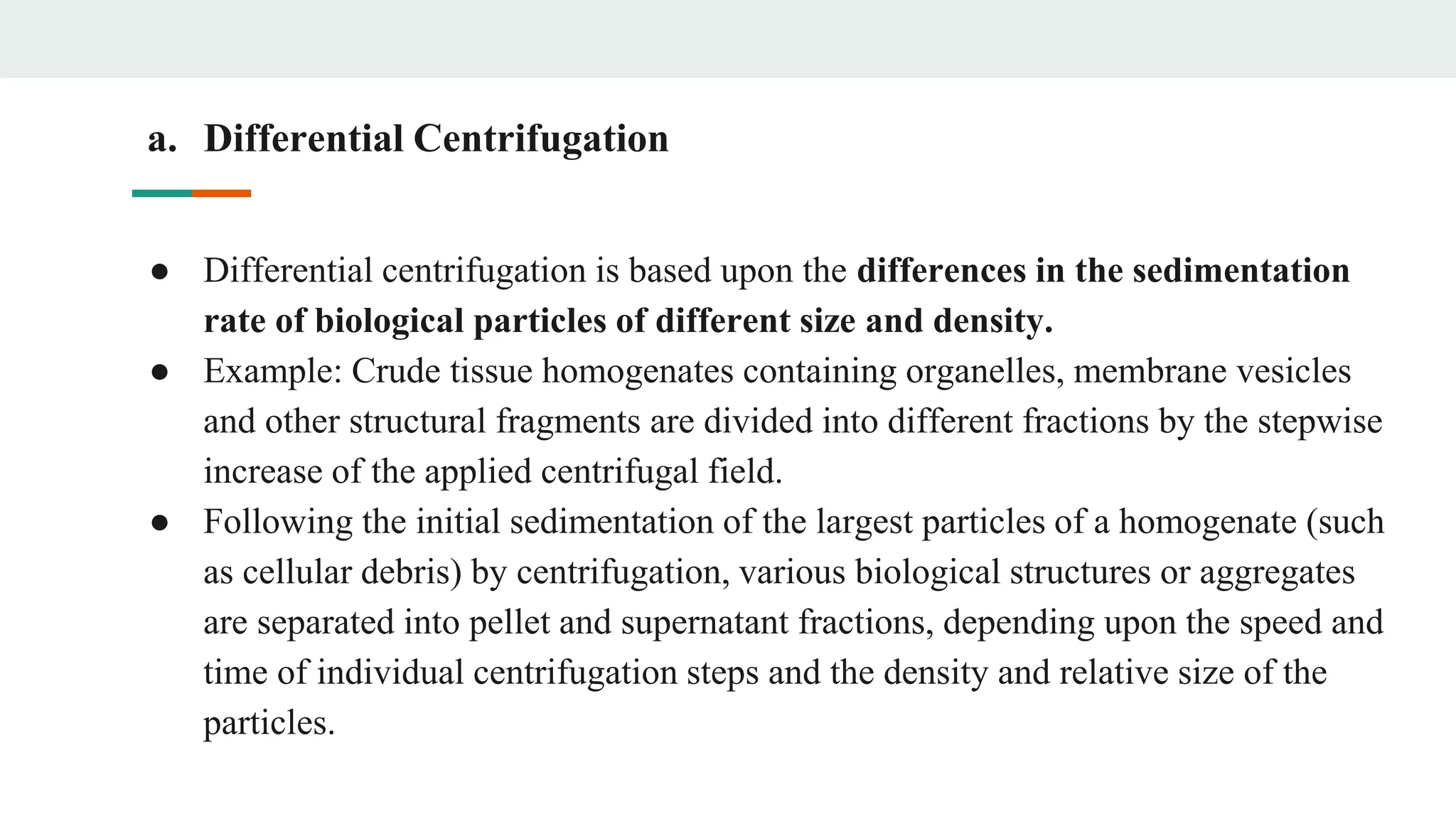
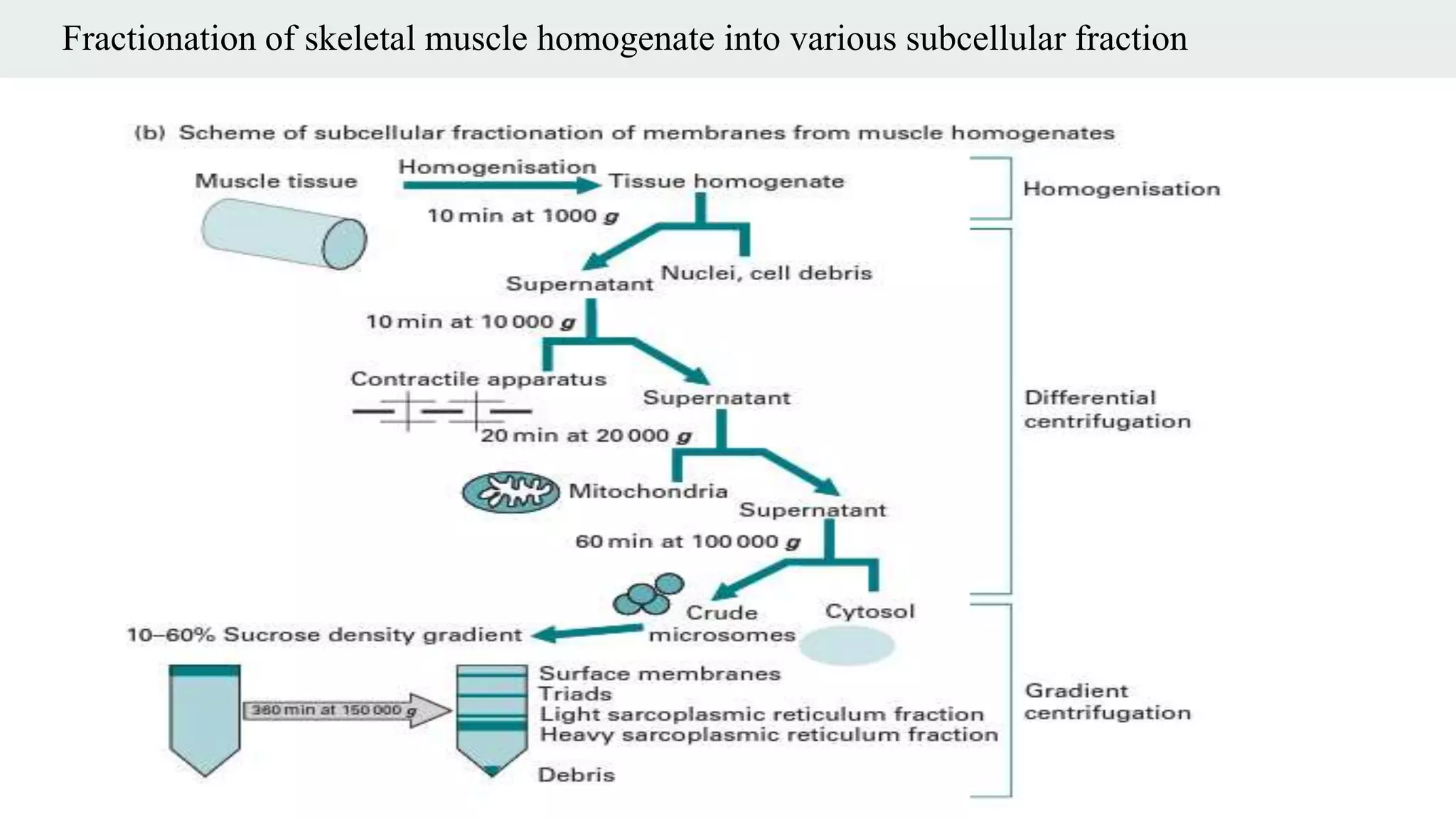
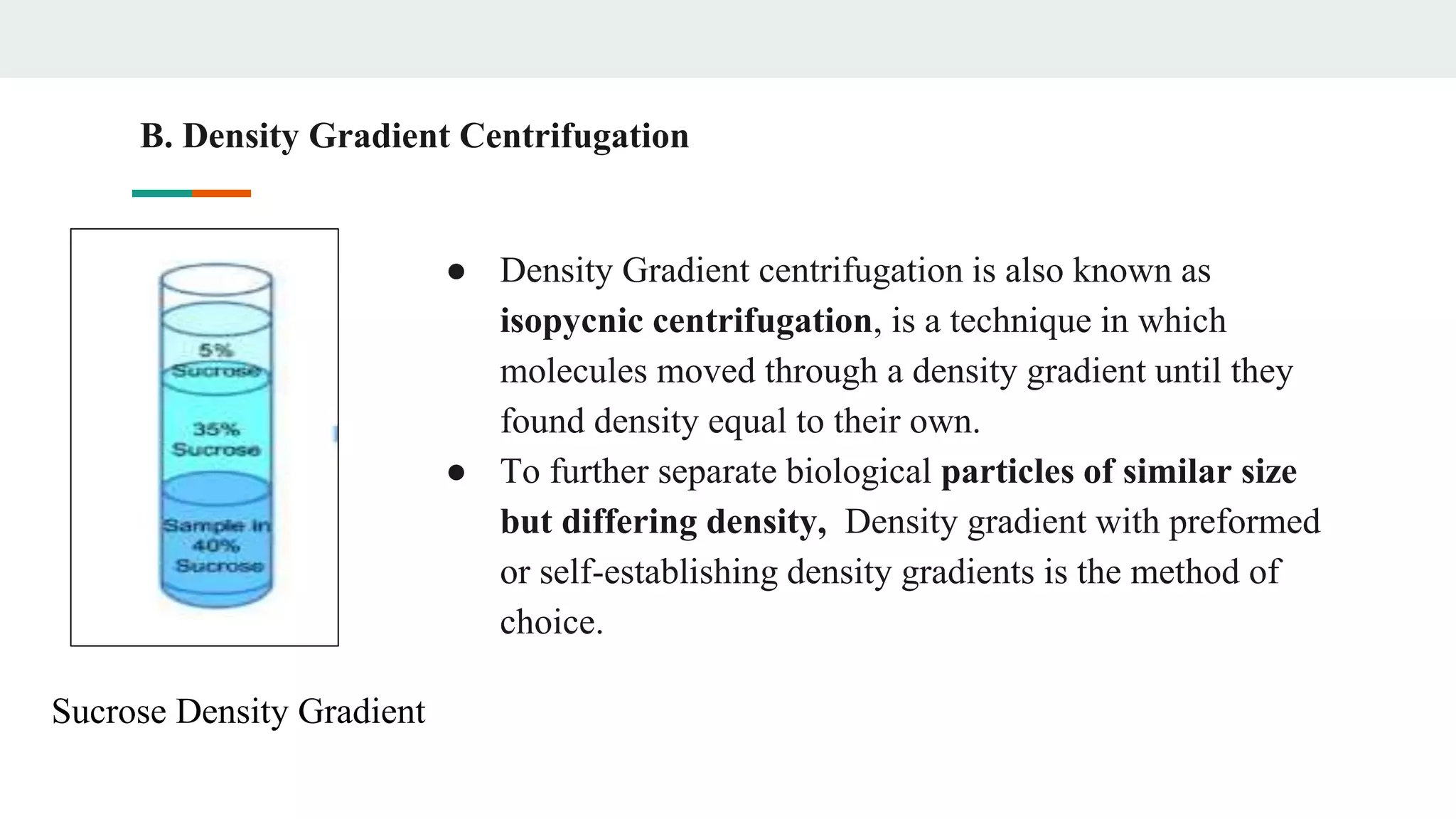
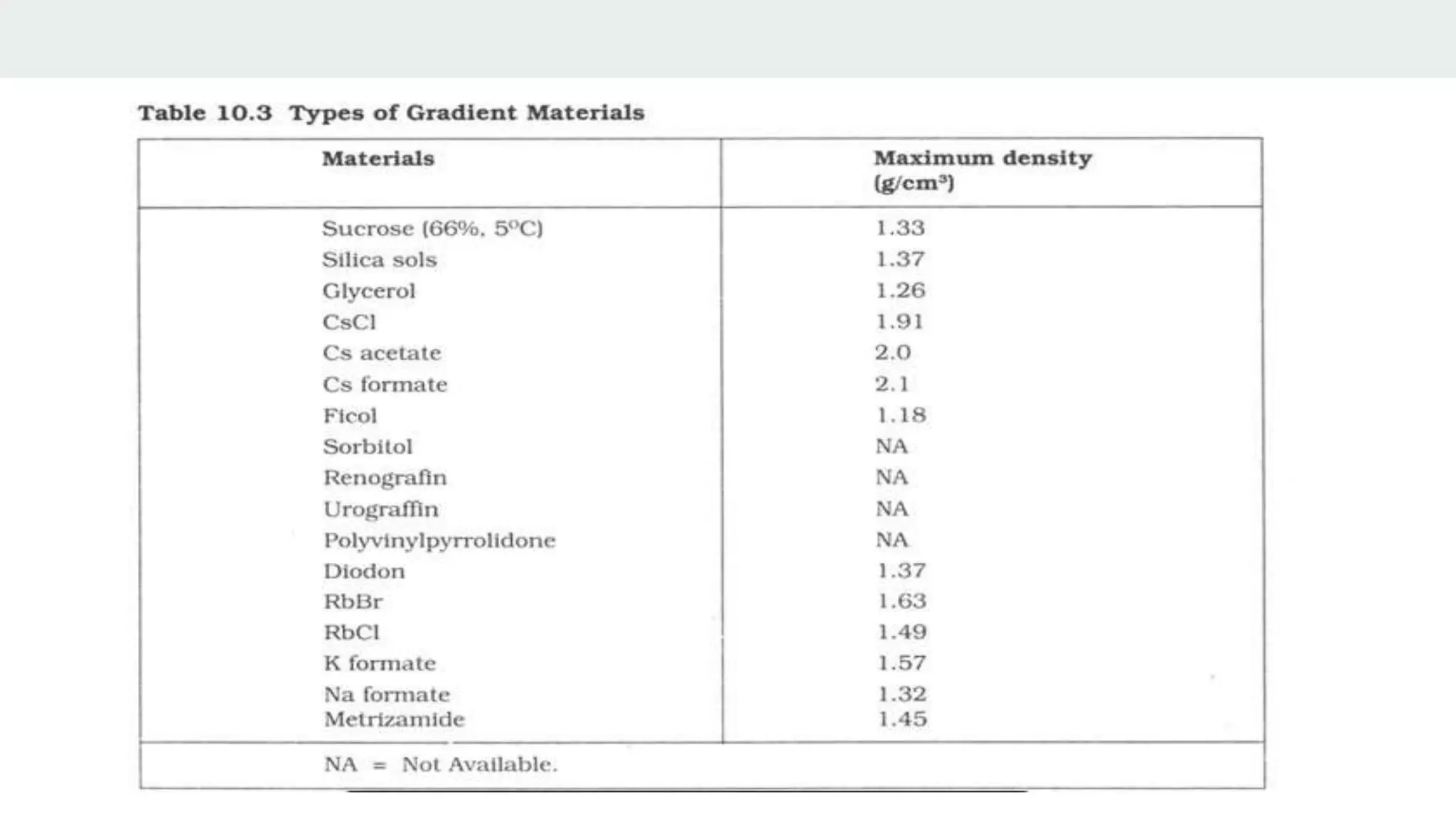
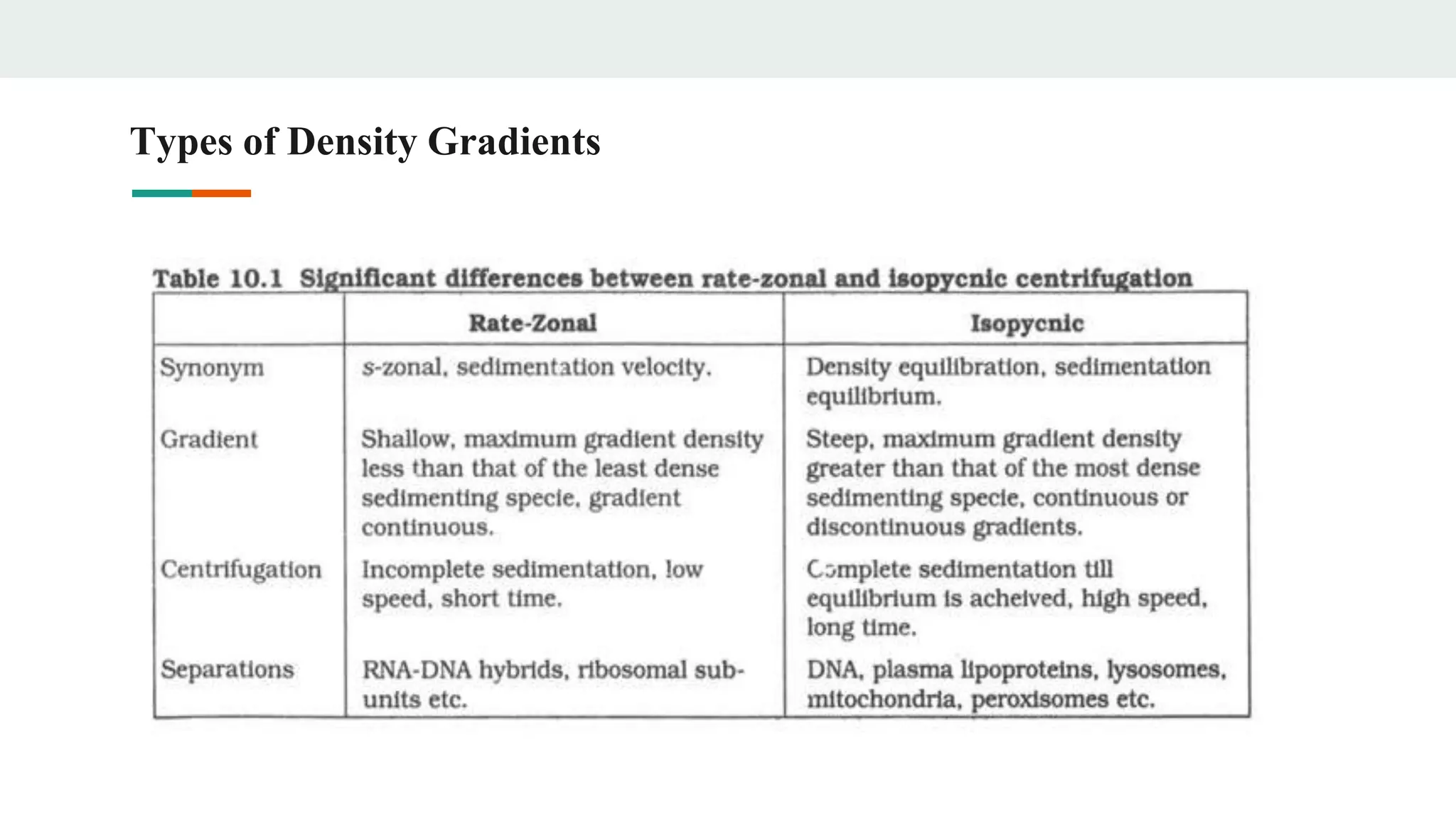
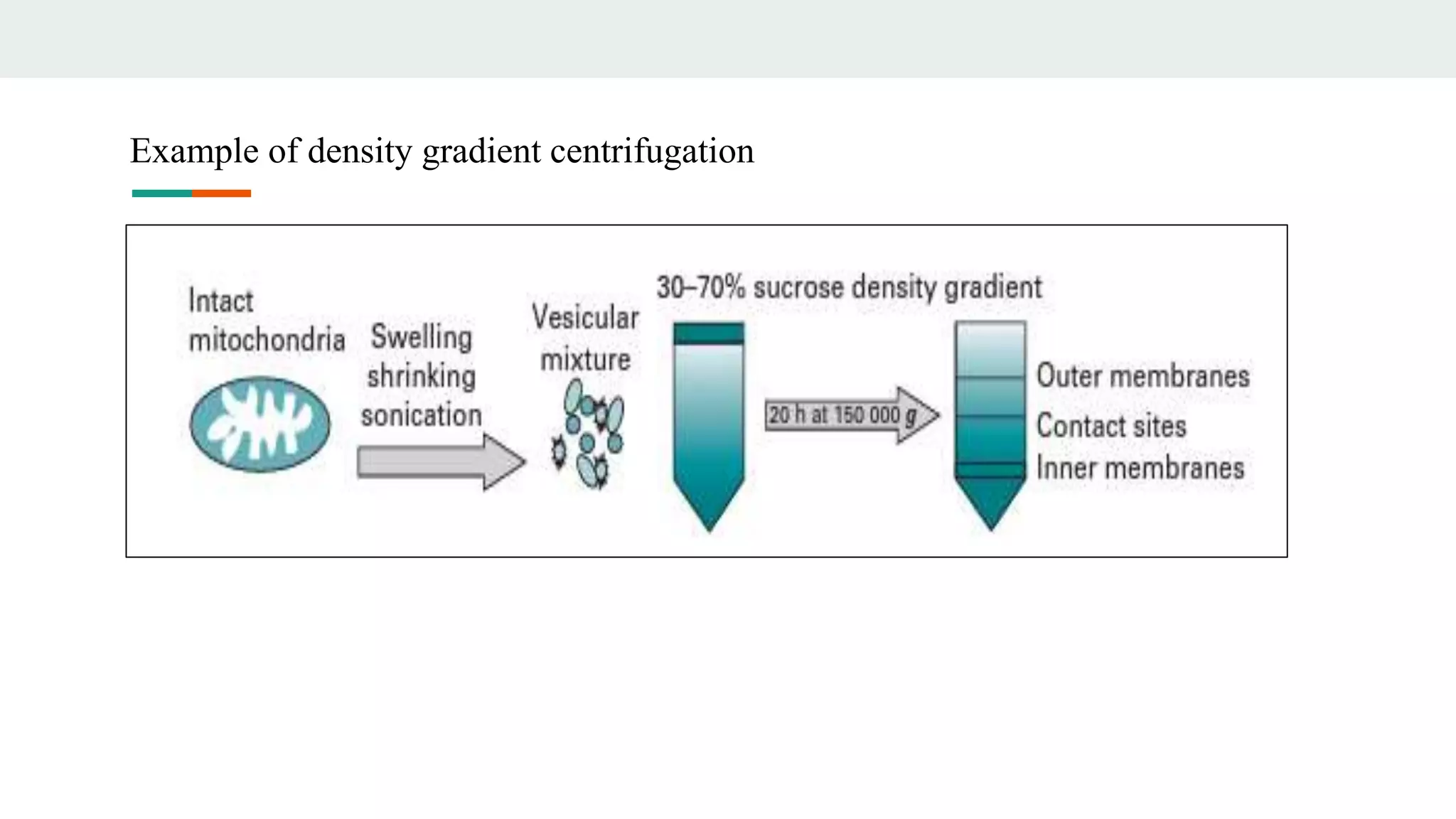
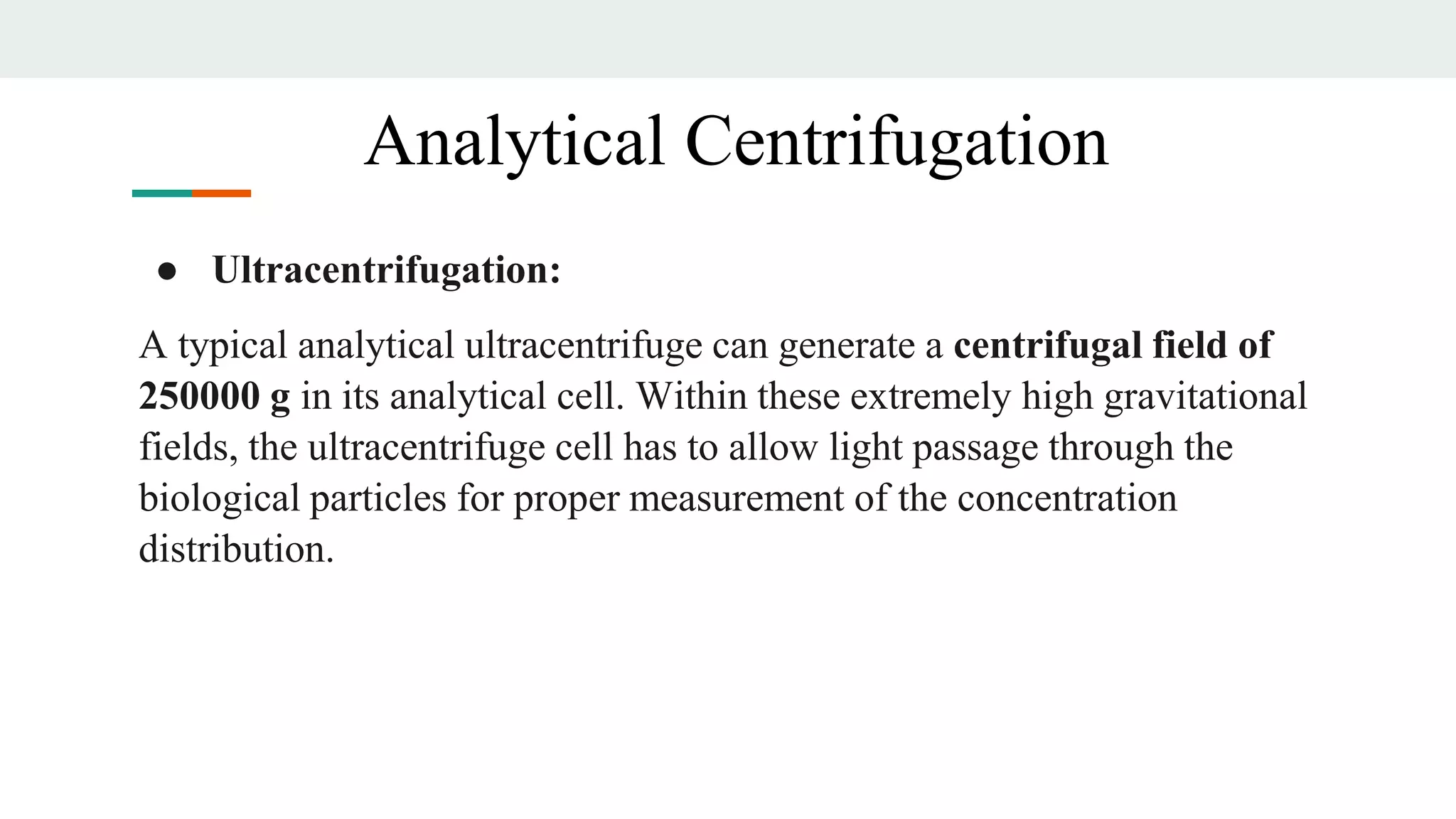
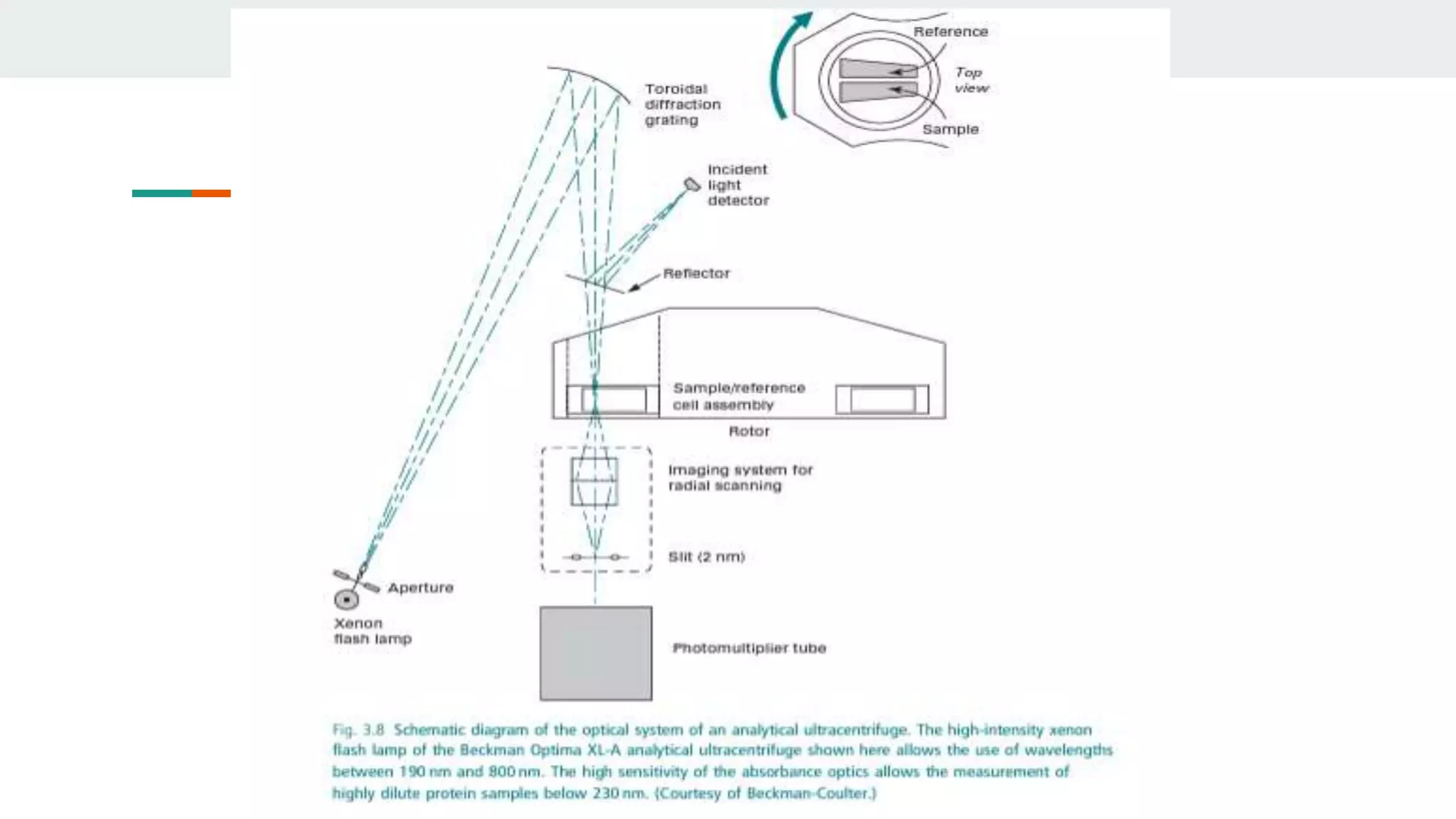
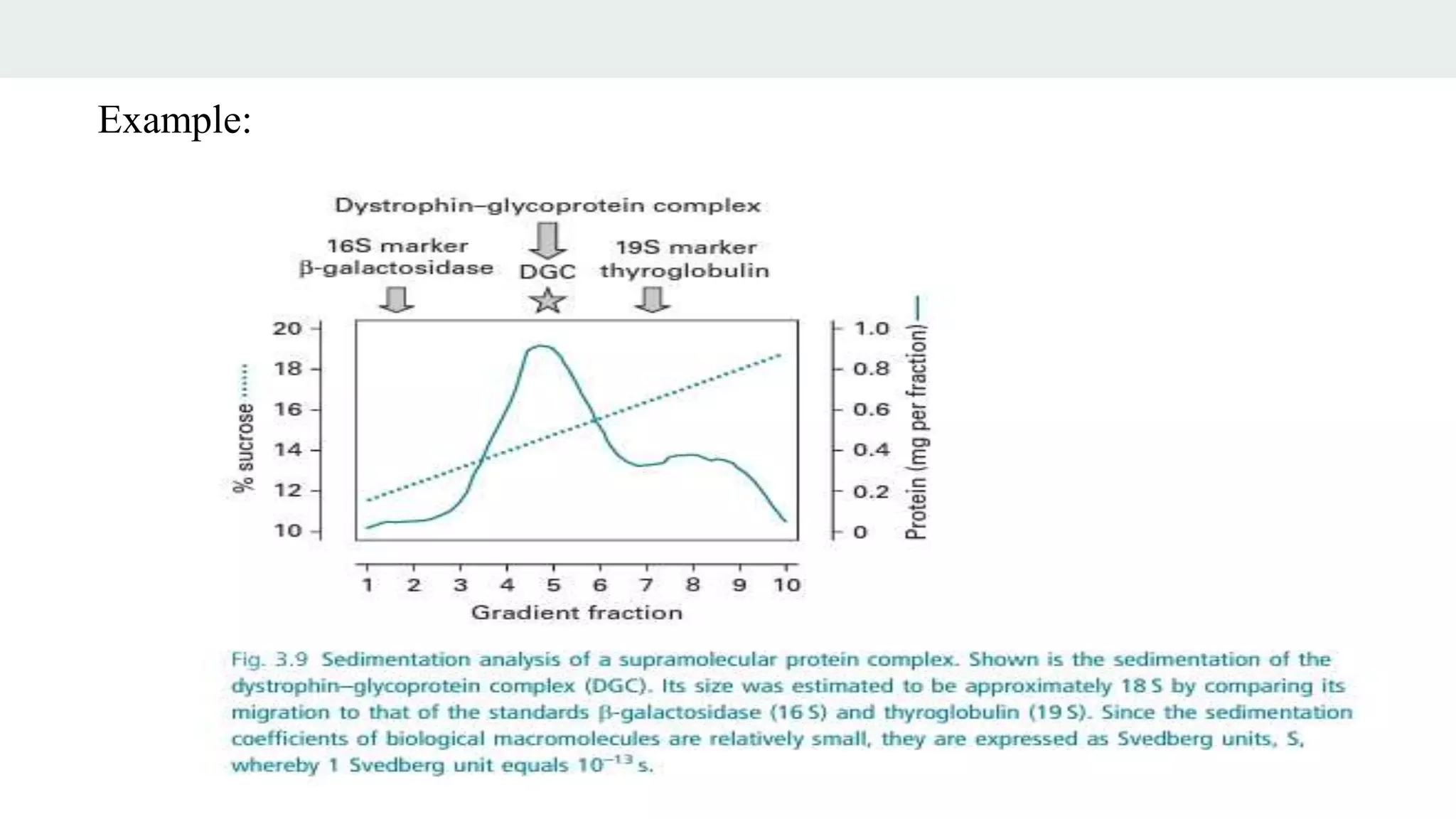
Centrifugation is a technique that utilizes centrifugal force to separate particles in fluids based on density and size, with applications ranging from cell collection to molecular weight measurement. The document outlines various types of centrifugation methods, including differential and density gradient centrifugation, as well as the principles behind sedimentation rates affected by particle characteristics and medium properties. Analytical ultracentrifugation is highlighted for its ability to measure molecular properties and purity of biological molecules with precision.