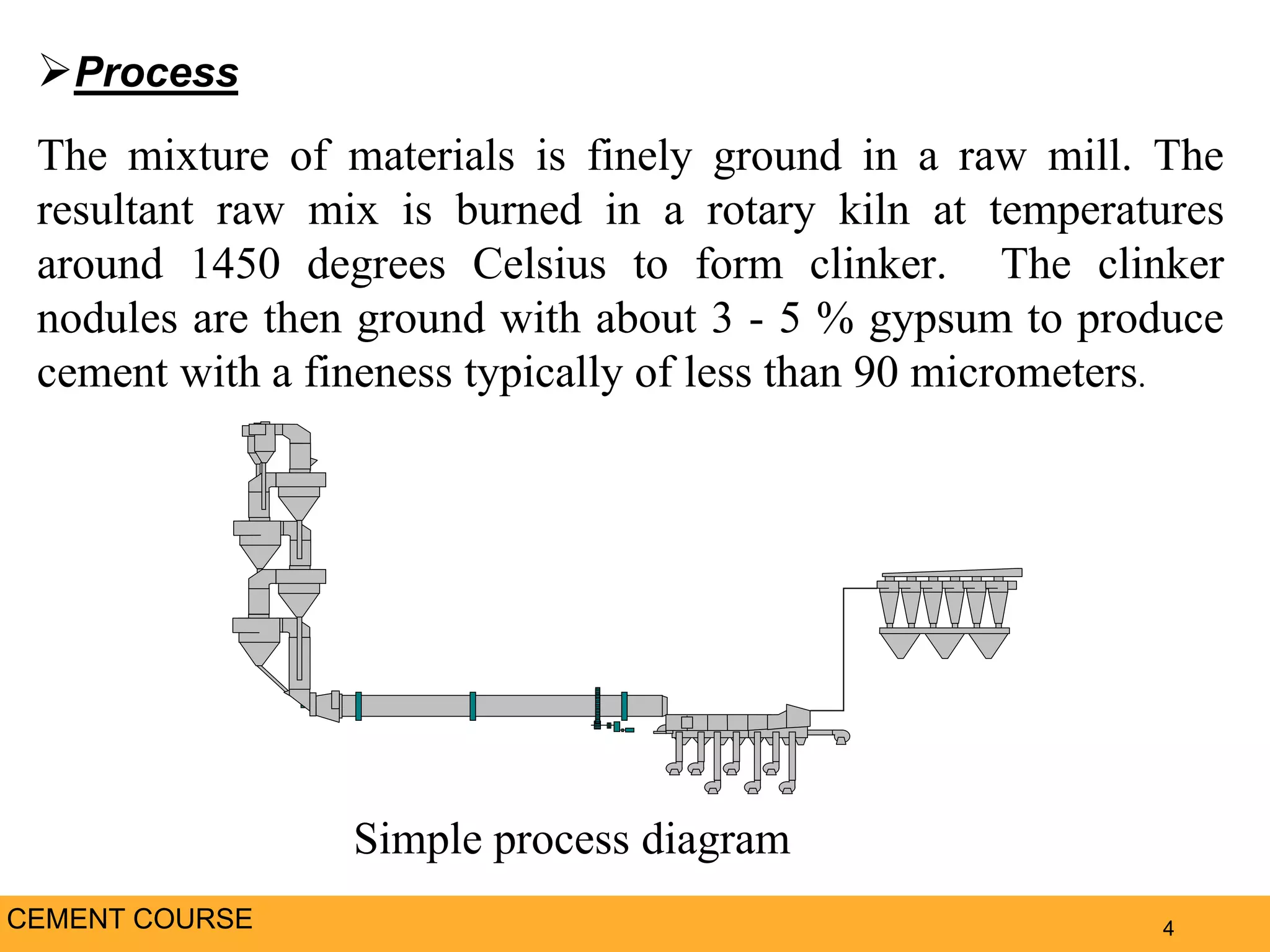
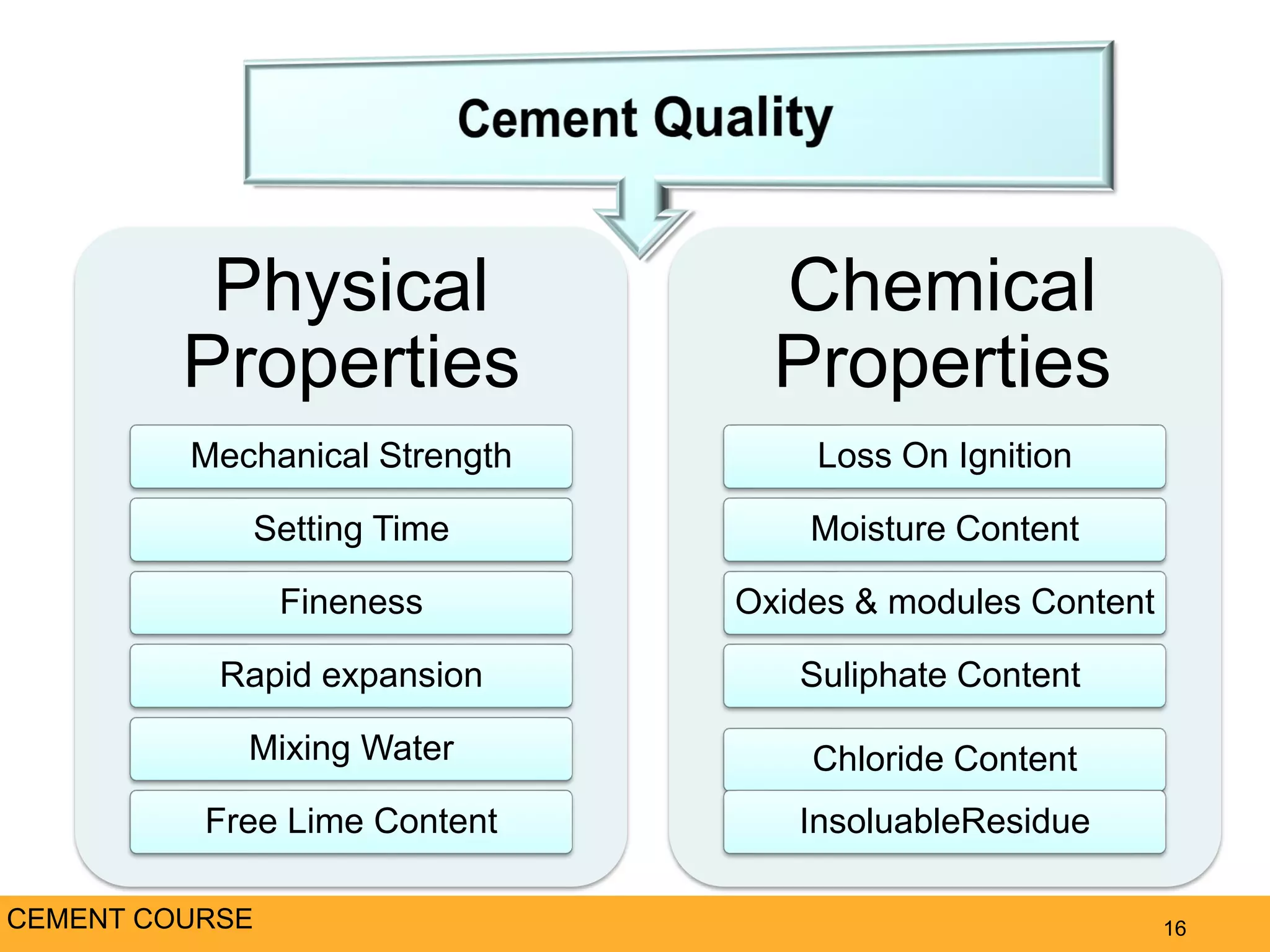
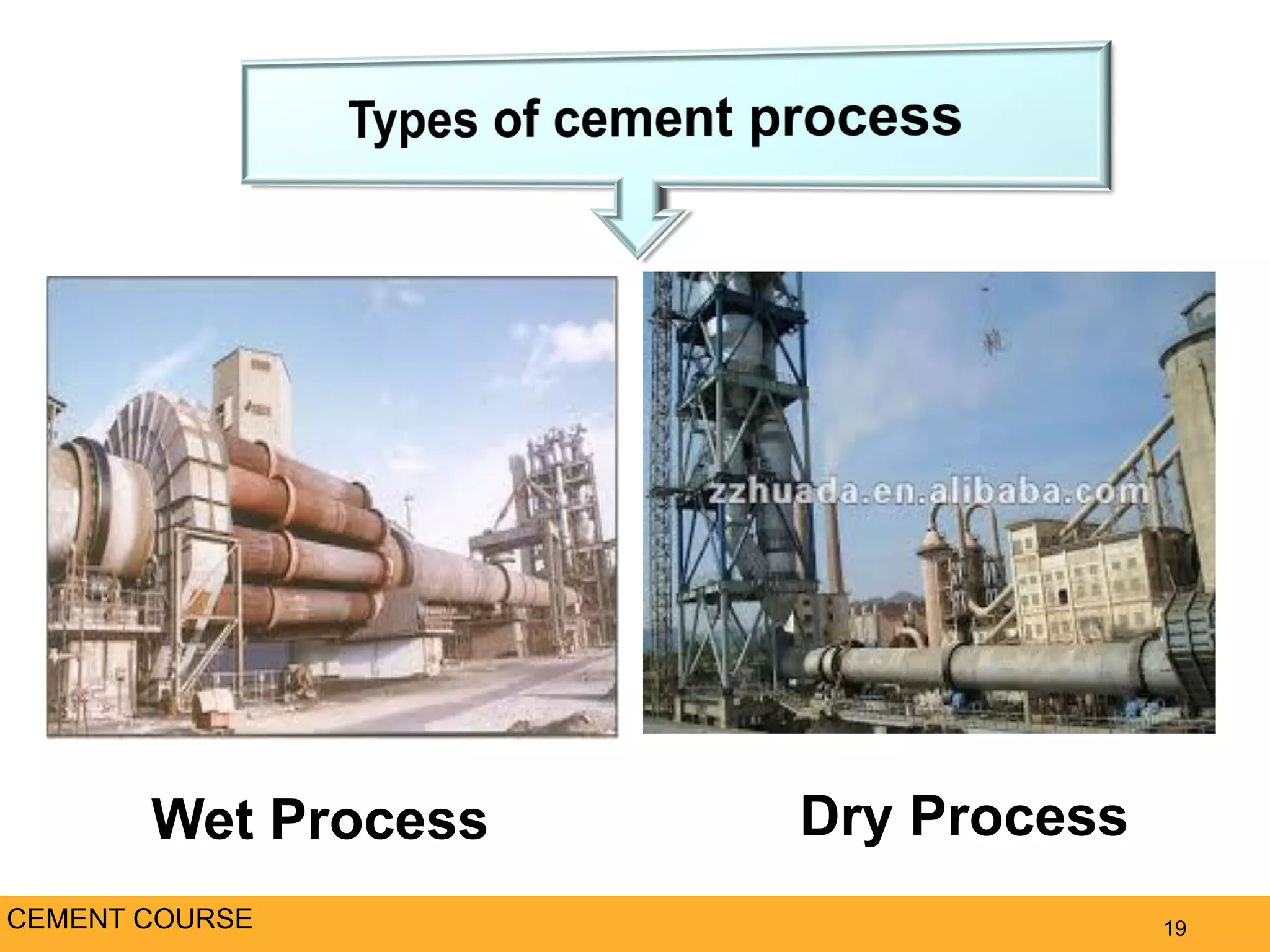
The document provides an overview of cement production. It discusses the history of cement, which was first invented by Egyptians and later refined by Greeks and Romans. The key raw materials used are limestone and materials containing clay and silica. Approximately 3,400 pounds of raw materials are needed to produce one ton of Portland cement. The mixture is ground, burned at high temperatures to form clinker, and then ground again with gypsum to produce cement. The document outlines the cement production process and discusses the chemistry and properties of cement, as well as the industry, environmental impacts, and alternatives.