Embed presentation
Download to read offline





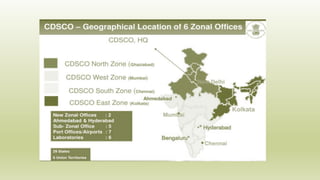





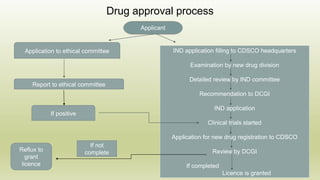




This document discusses the Central Drug Standard Control Organization (CDSCO) and State Licensing Authorities in India and their roles and responsibilities. CDSCO is responsible for approval of new drugs, clinical trials, laying down standards for drugs, control over imported and exported drugs. It has a head office in Delhi and zonal offices in major cities. State Licensing Authorities are responsible for issuing licenses to manufacturing and sale of drugs within a state. The document outlines the hierarchy, functions, and drug approval process of CDSCO as well as portals for online licensing.















