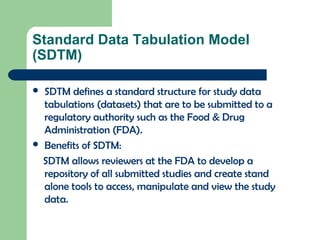
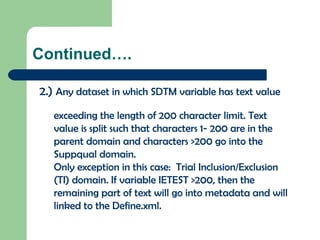
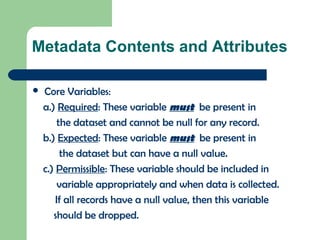
The document discusses the Combined Data Interchange Standard Consortium (CDISC) and its Standard Data Tabulation Model (SDTM). CDISC develops standards to support clinical research data exchange and submission. SDTM defines a standard structure for study data tabulations submitted to regulators. The document outlines key aspects of SDTM including its implementation guide, fundamentals, observation classes, special purpose domains, trial design model, relationship datasets, metadata, controlled terminology, and date/time variables.