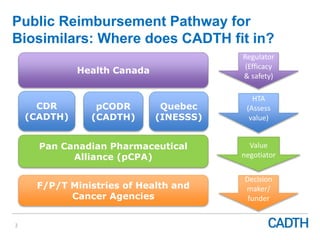
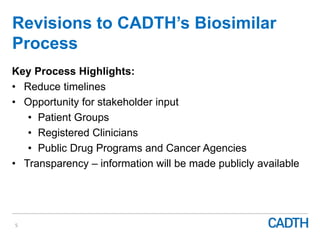
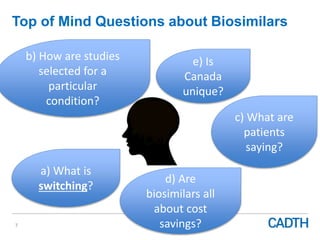
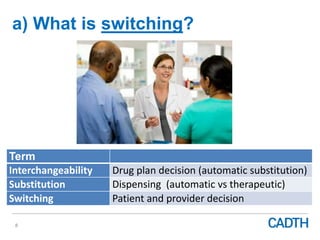
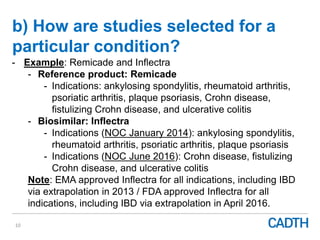
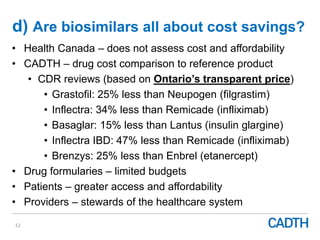
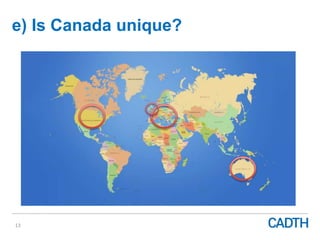
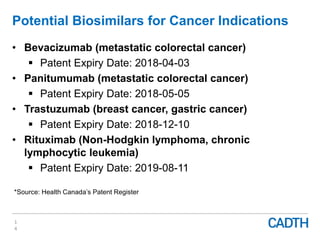
The webinar discusses the public reimbursement pathway for biosimilars in Canada and outlines key questions concerning switching, study selection, patient perspectives, cost savings, and Canada's unique position. It emphasizes CADTH's role in streamlining the biosimilars review process to enhance patient access, alongside considerations of clinical safety and efficacy. Potential biosimilars for cancer indications are also highlighted, alongside their respective patent expiry dates.