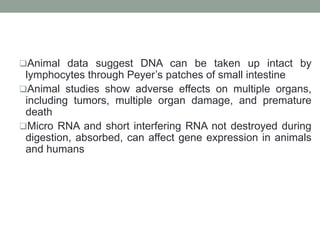
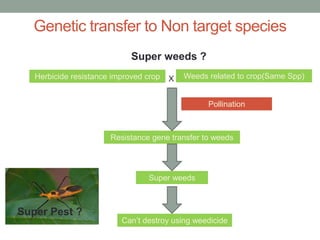
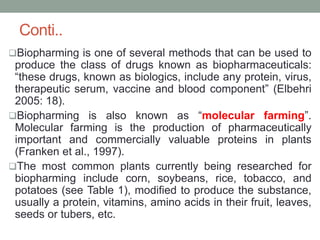
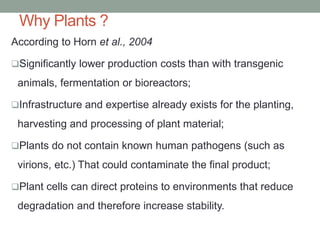
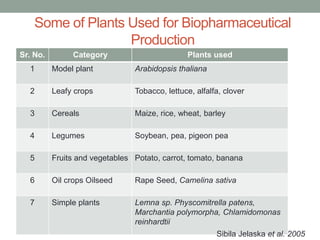
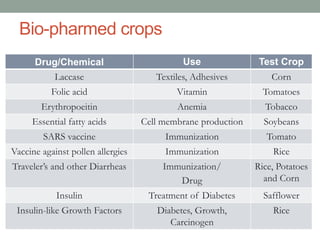
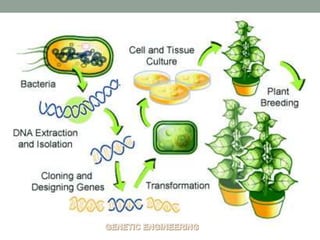
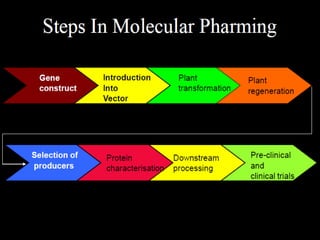
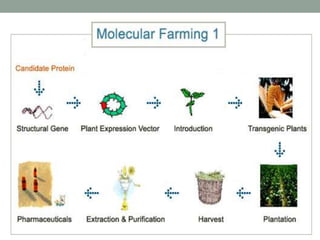
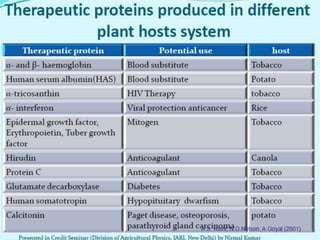
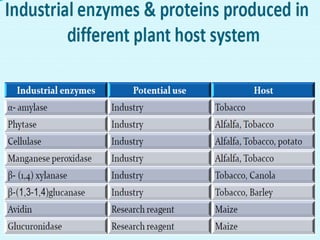
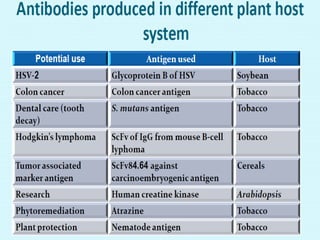
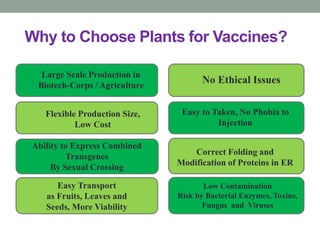
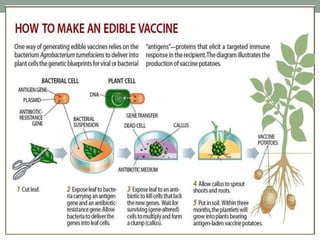
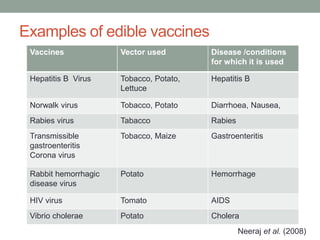
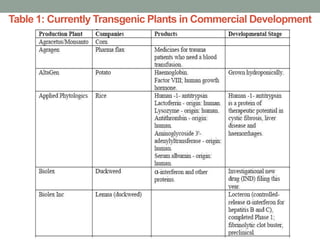
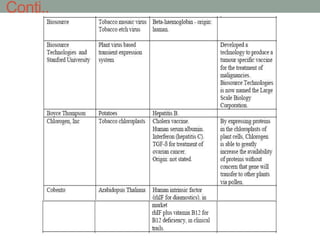
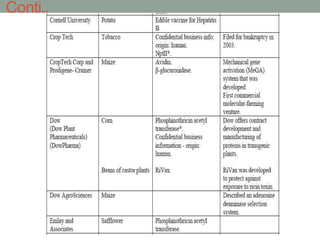
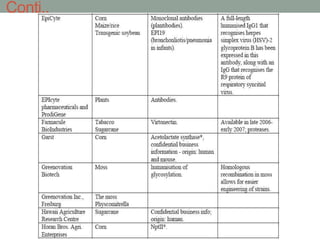
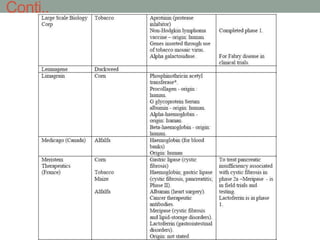
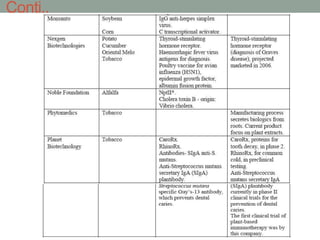
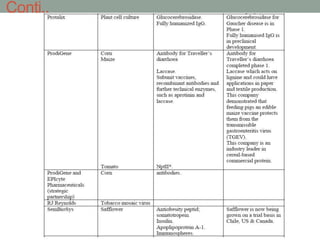
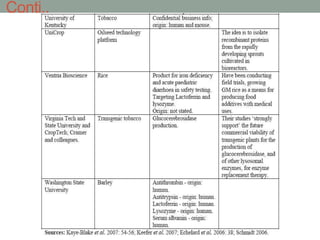
Plant biopharming involves genetically modifying crops to produce pharmaceuticals for human use, offering cost-effective production and diverse applications, from vaccines to cancer treatments. The method leverages existing agricultural infrastructure, reduces contamination risks, and has significant potential for addressing global health challenges, especially in developing countries. However, concerns regarding genetic risks, regulatory issues, and public health implications remain critical.
















![Allergenicity in India
In India, hundreds of laborers picking cotton and working in cotton ginning
factories developed allergic reactions when handling the BT cotton. This didn’t
happen with the non-Bt varieties. [Ashish Gupta et. al., “Impact of Bt Cotton on
Farmers’ Health (in Barwani and Dhar District of Madhya Pradesh),”
Investigation Report, Oct–Dec 2005]
Hospital records: “ Show that victims of itching have increased massively this
year, and all of them are related to BT cotton farming.” [The Sunday Indian,
10/26/08]
Itching all over the body,
eruptions, wounds,
discoloration](https://image.slidesharecdn.com/kkcbiopharming-150308035253-conversion-gate01/85/Biopharming_Designer_Crops-43-320.jpg)