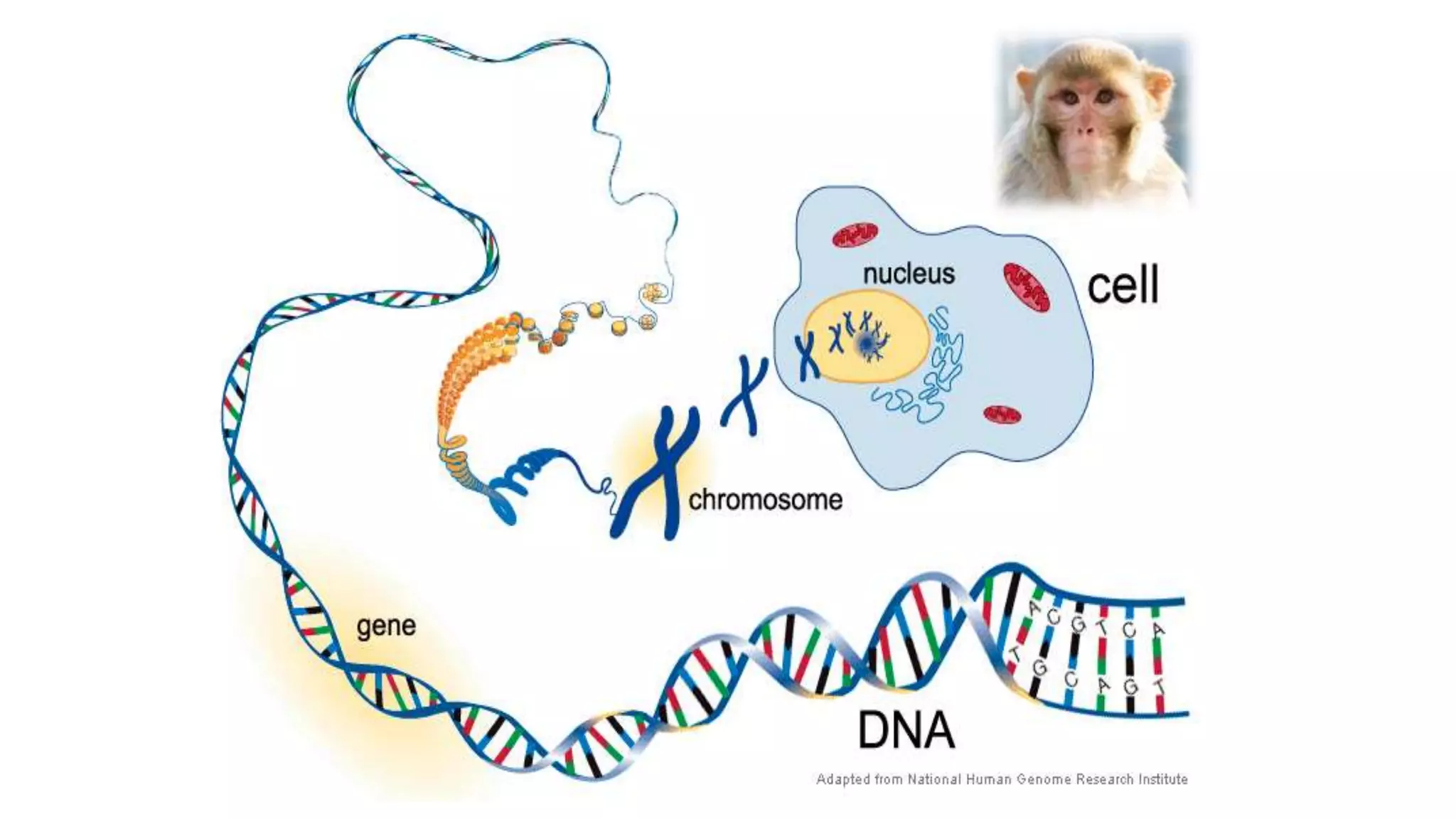
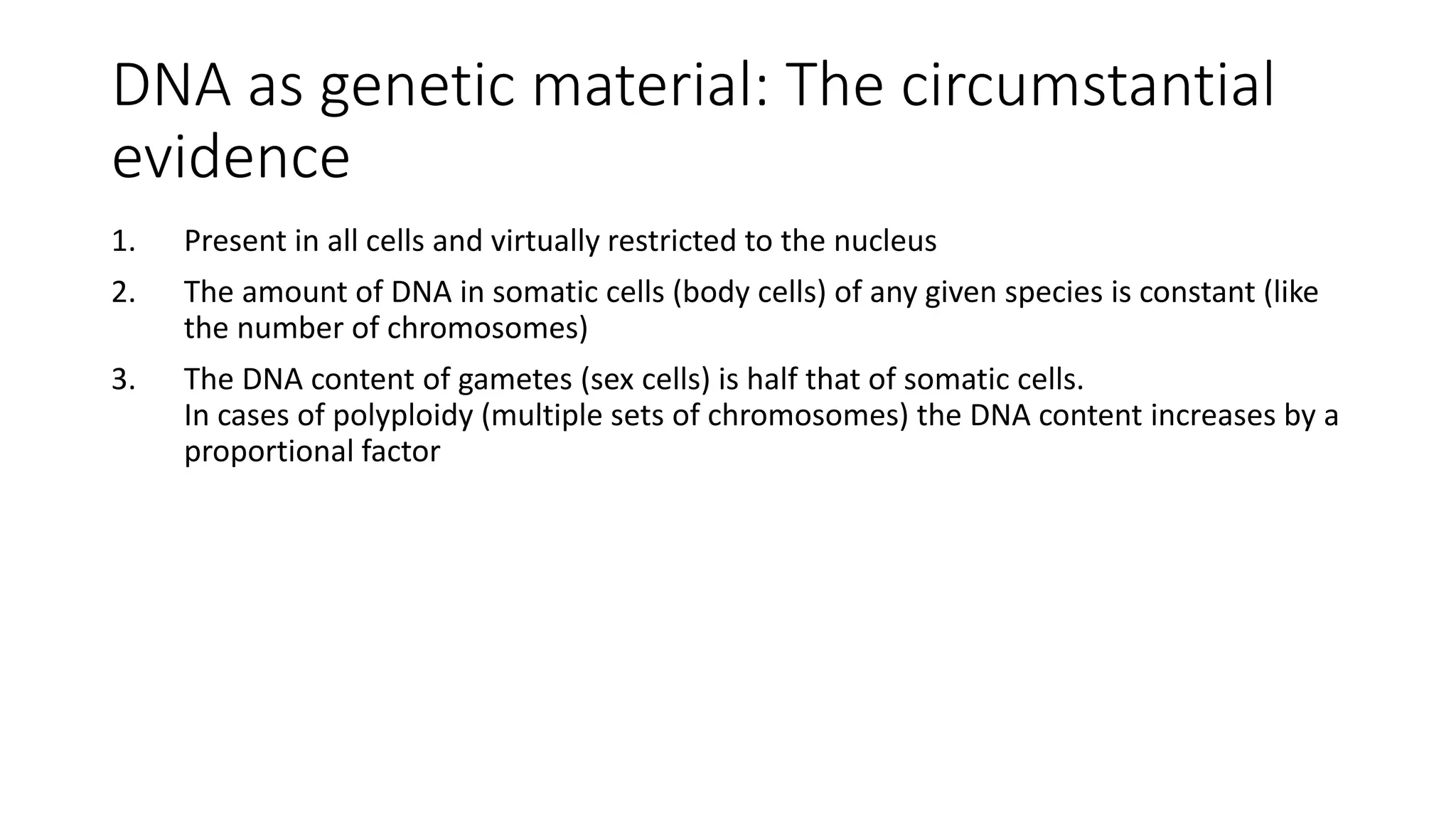
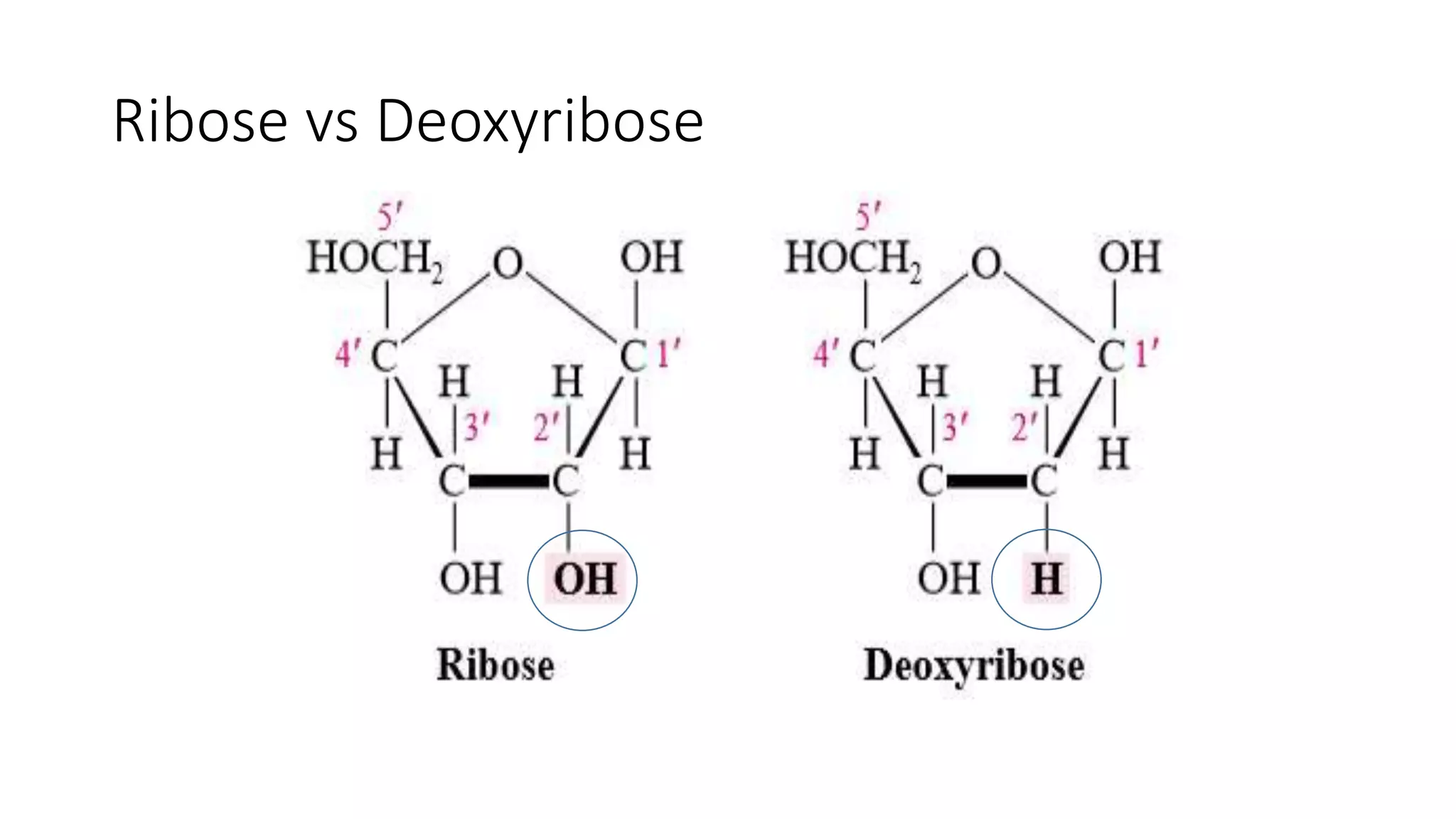
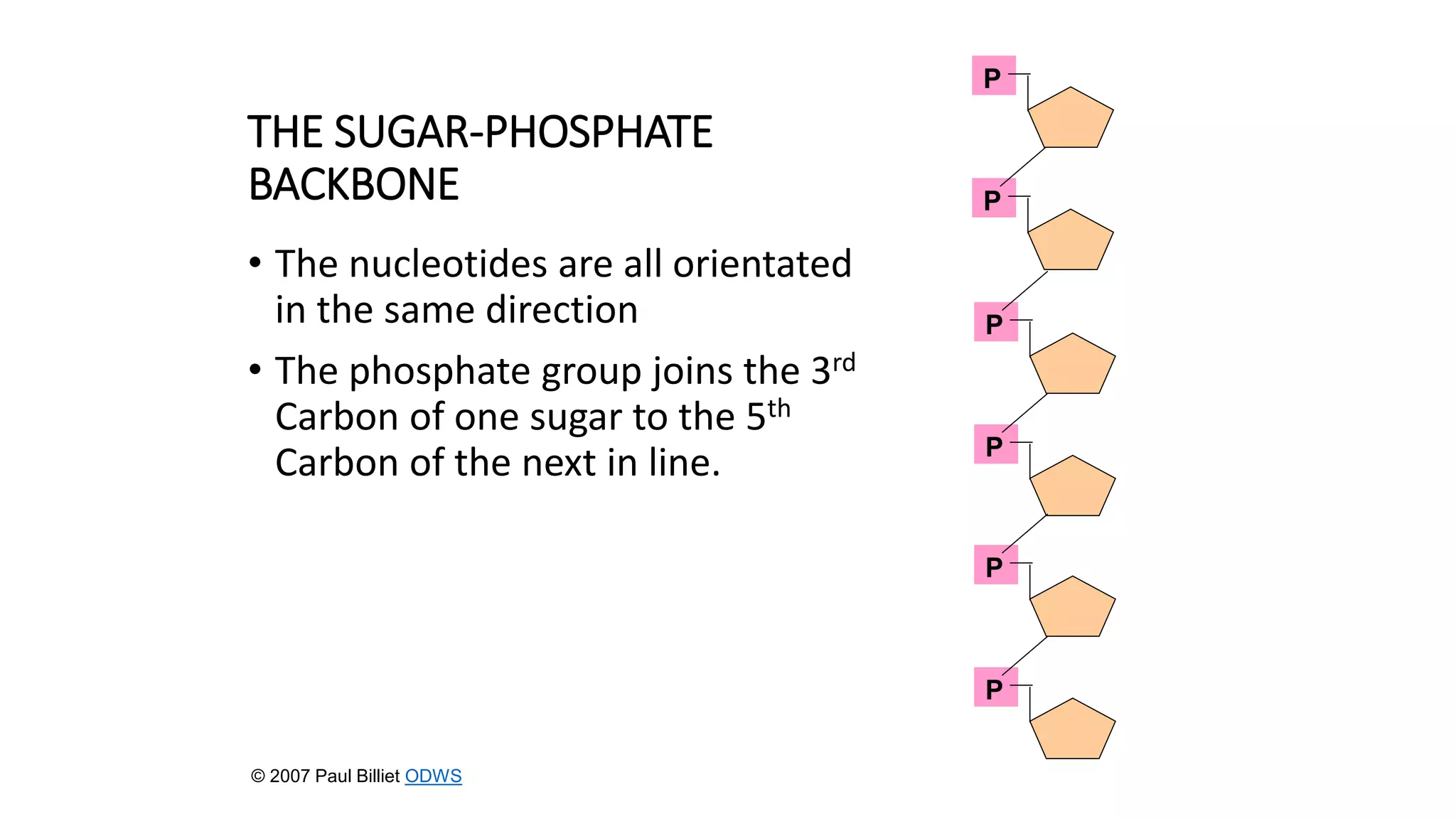
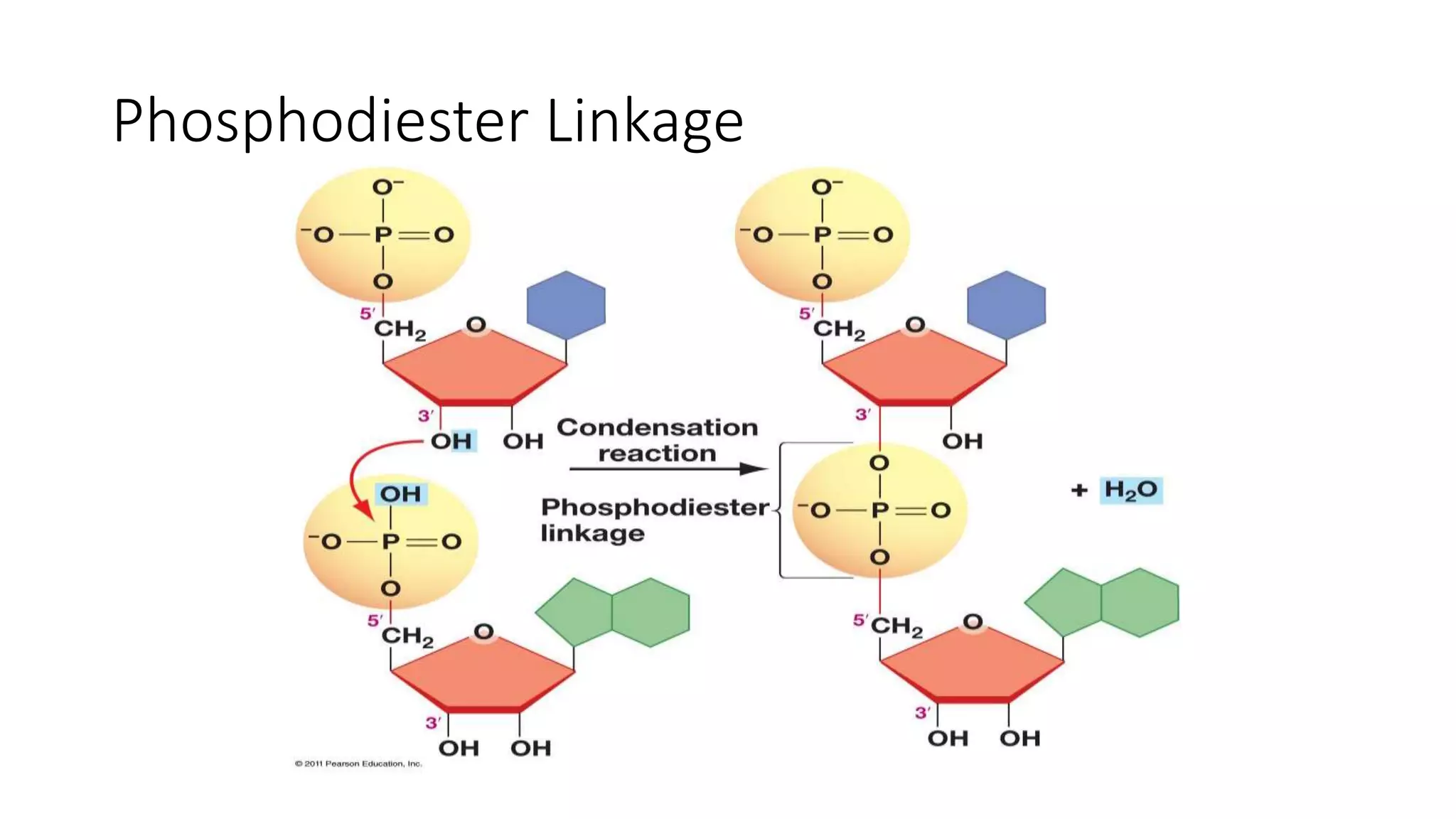
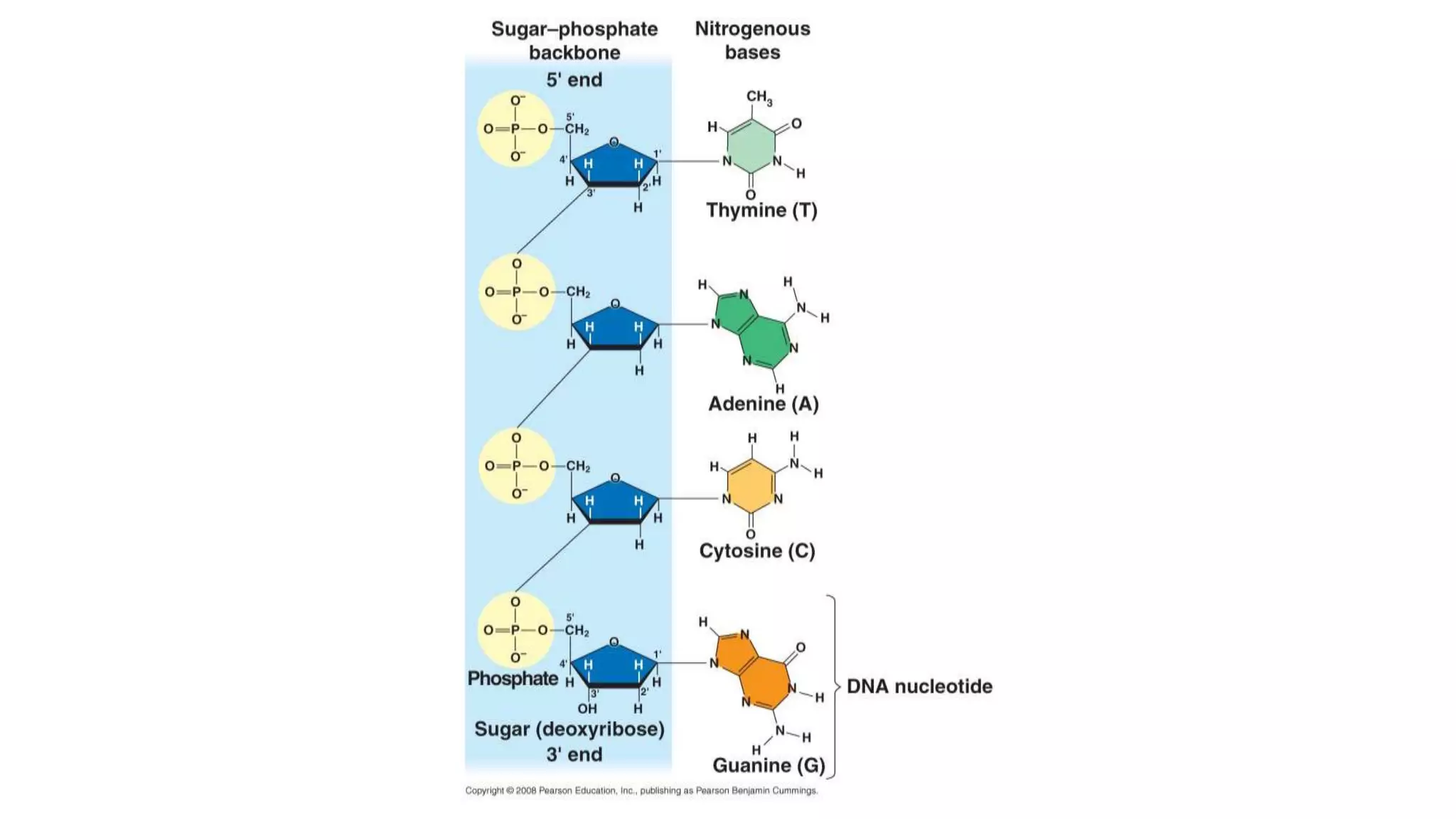
DNA sequencing involves analyzing the order of nucleotides in DNA. There are two types of nucleic acids: DNA and RNA. DNA is found in the nucleus and the amount is constant in somatic cells but half in gametes. Nucleotides are the subunits of DNA and RNA and contain a phosphate group, a sugar (ribose or deoxyribose), and a nitrogenous base. A nucleoside contains a sugar and base while a nucleotide additionally contains a phosphate group. The structures of nucleotides involve the phosphate group bonding to the sugar, the sugar-phosphate backbone formed by phosphodiester linkages between sugars, and various nitrogenous bases including purines and pyrimidines bonding to the sugar.