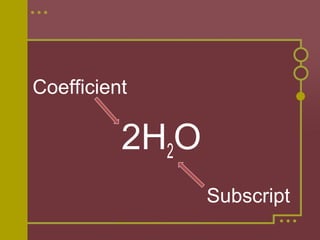
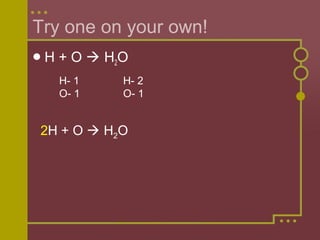
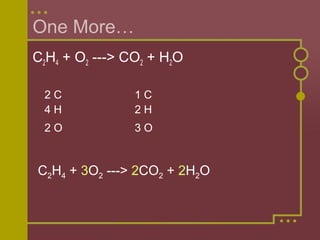
The document discusses how to balance chemical equations by ensuring the total number of atoms on both sides of the equation are equal. It explains that coefficients are used to represent the number of molecules in a reaction, while subscripts indicate the number of atoms in a molecule. The steps to balance an equation are to: 1) Write the unbalanced equation, 2) Count the atoms on each side, 3) Use coefficients to make the atoms equal on both sides. Examples are provided to demonstrate balancing equations through adjusting coefficients and verifying the balanced atoms.