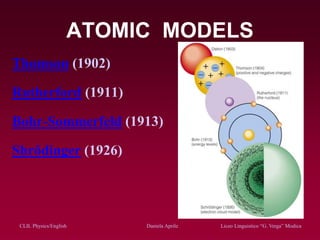
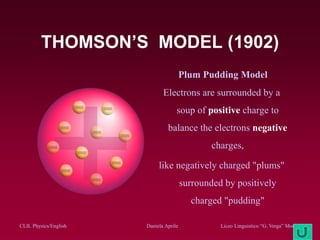
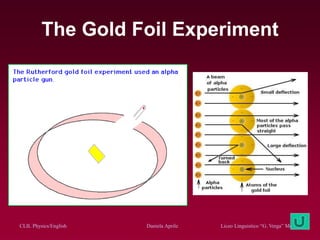
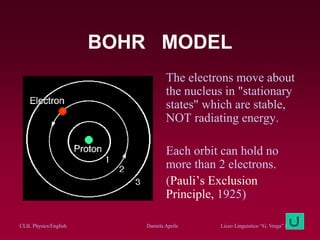
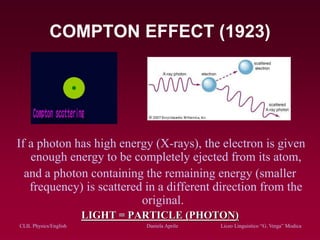
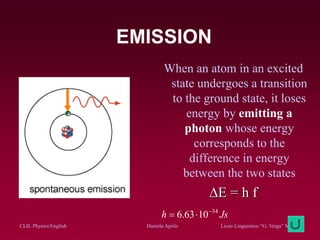
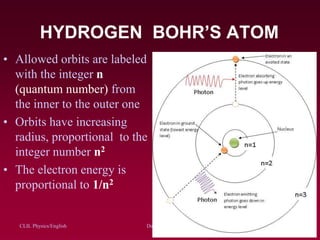
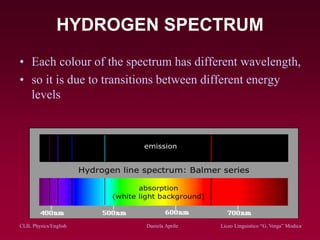
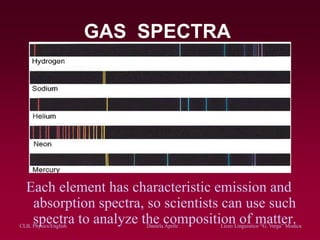
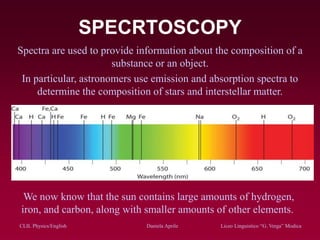
The document discusses atomic spectra and models of the atom, focusing on key historical discoveries and various atomic models, including Thomson's plum pudding model, Rutherford's model, and Bohr's model. It explains the relationship between atomic structure and spectral emissions, emphasizing the significance of photons in energy transitions of electrons. The document concludes by acknowledging limitations of Bohr's model and hints at advanced concepts in quantum physics for further study.