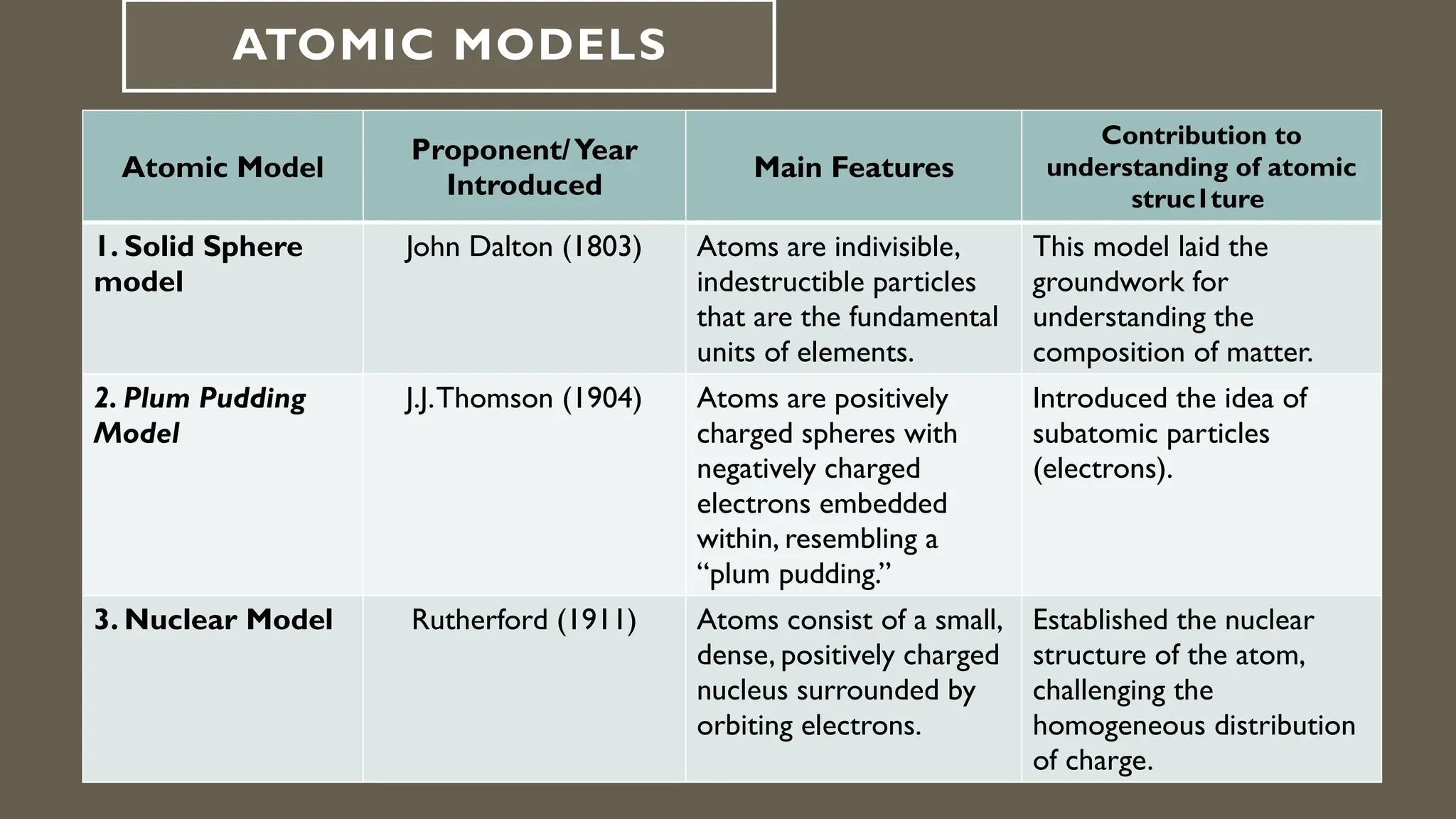
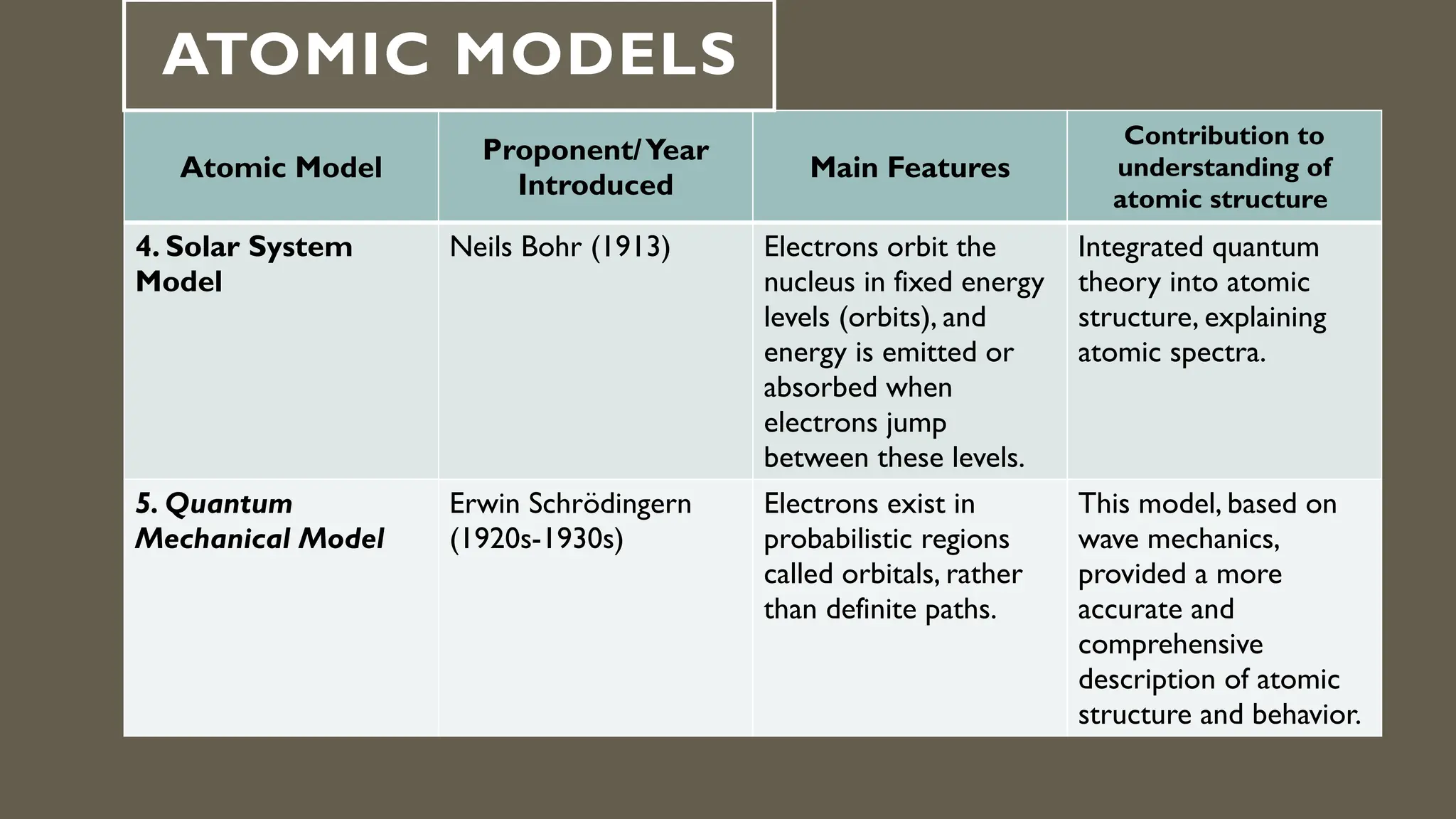
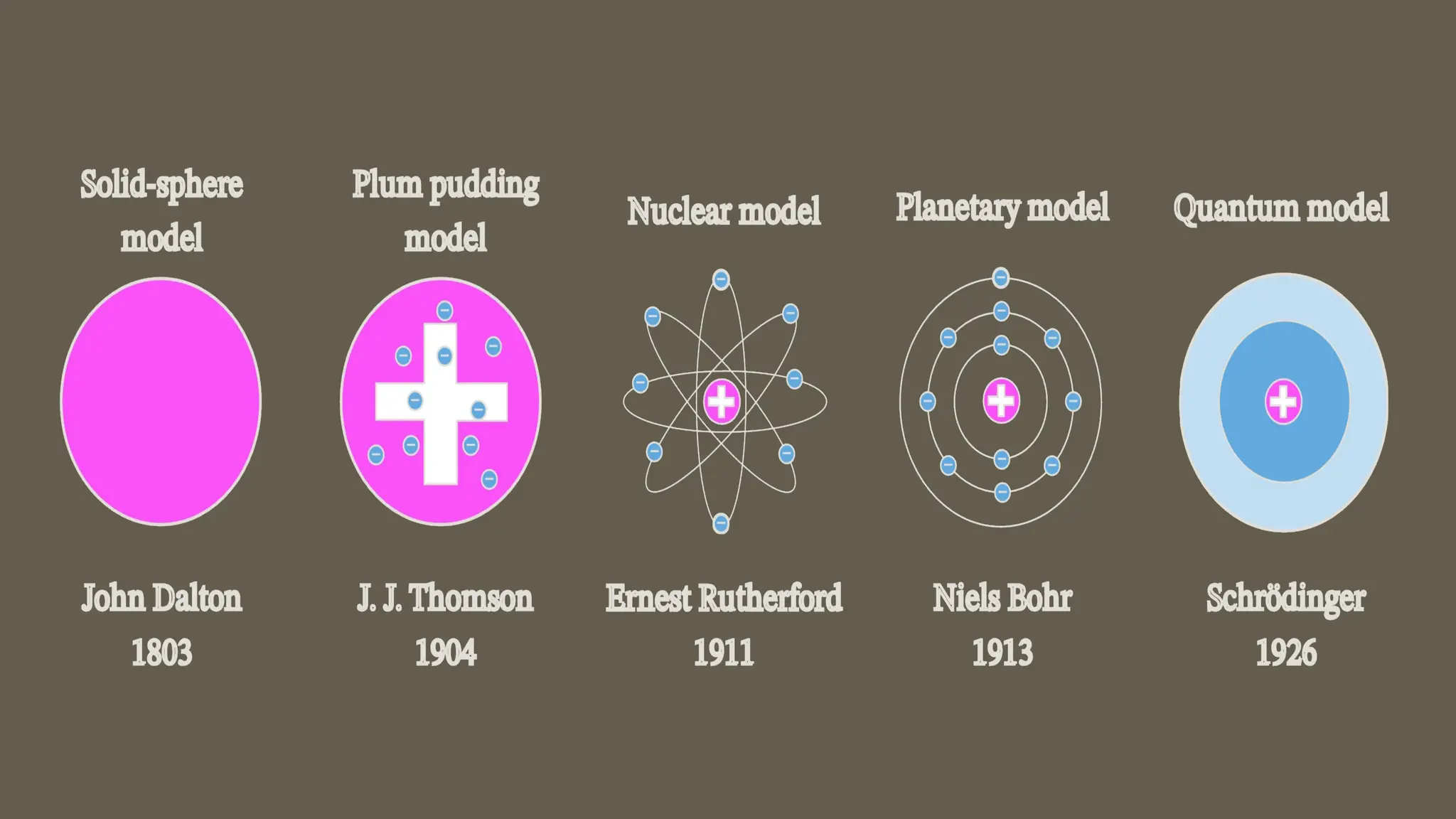
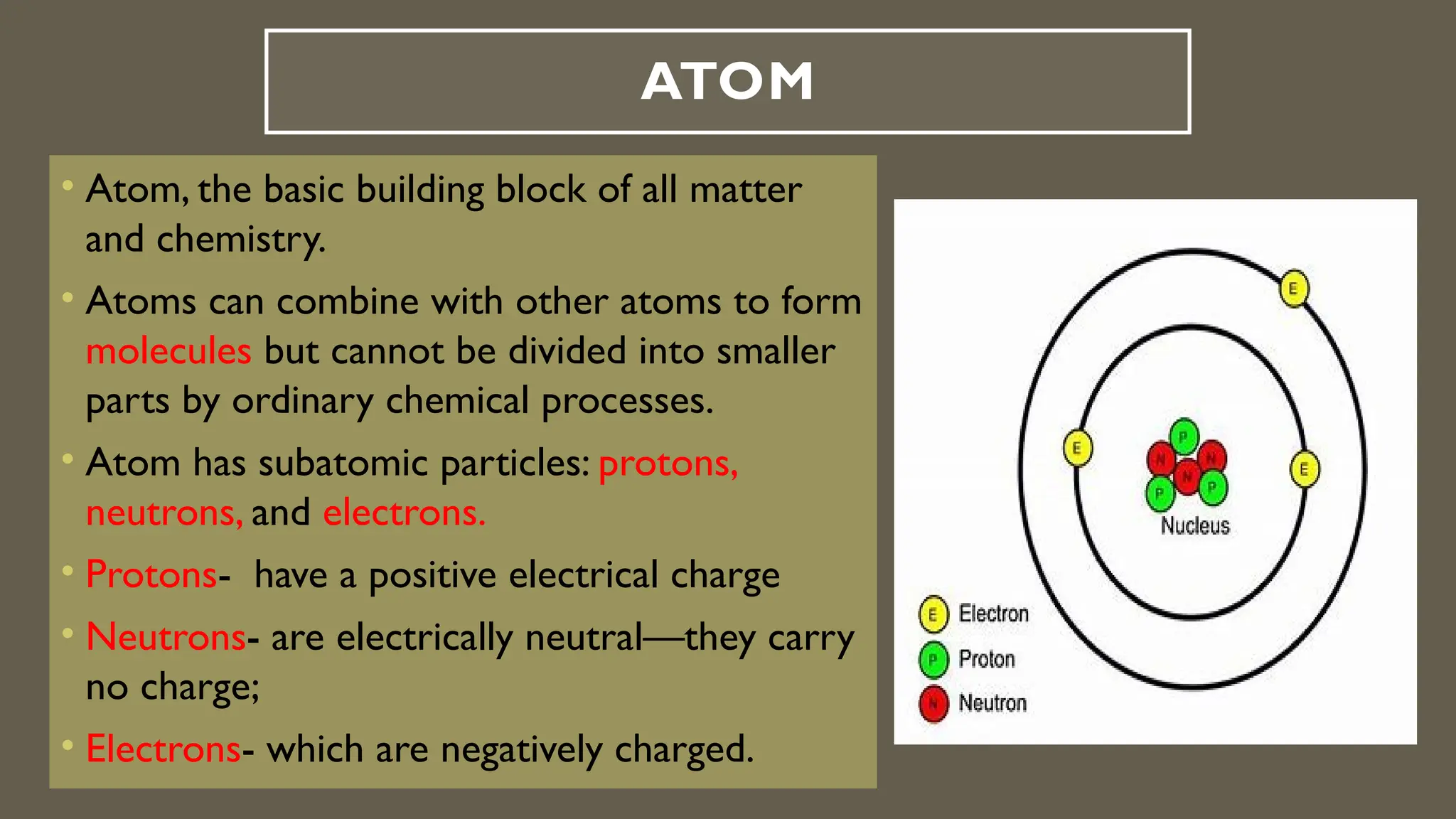
This document outlines the evolution of atomic models from Dalton's solid sphere model in 1803 to Schrödinger's quantum mechanical model in the 1920s-1930s. Each model contributed significantly to the understanding of atomic structure, introducing concepts like subatomic particles, nuclear structure, and probabilistic electron behaviors. Additionally, it discusses the particle model of matter, emphasizing how atoms and molecules interact and the motion of particles in different states of matter.