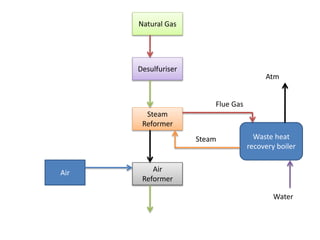
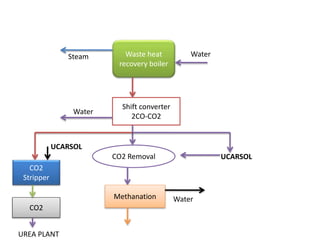
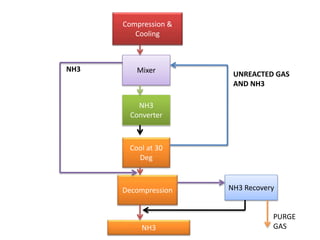
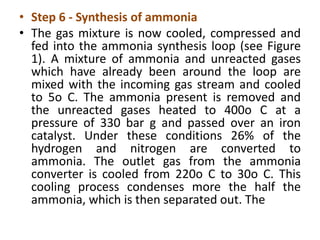
The ammonia manufacturing process involves 6 key steps:
1) Hydrogen is produced from natural gas through steam reforming.
2) Nitrogen from air is added to the synthesis gas.
3) Carbon monoxide is removed through a water gas shift reaction.
4) Water is removed by condensation.
5) Carbon dioxide is removed using an MDEA solution.
6) The purified gas mixture is compressed and catalyzed over iron to produce ammonia.