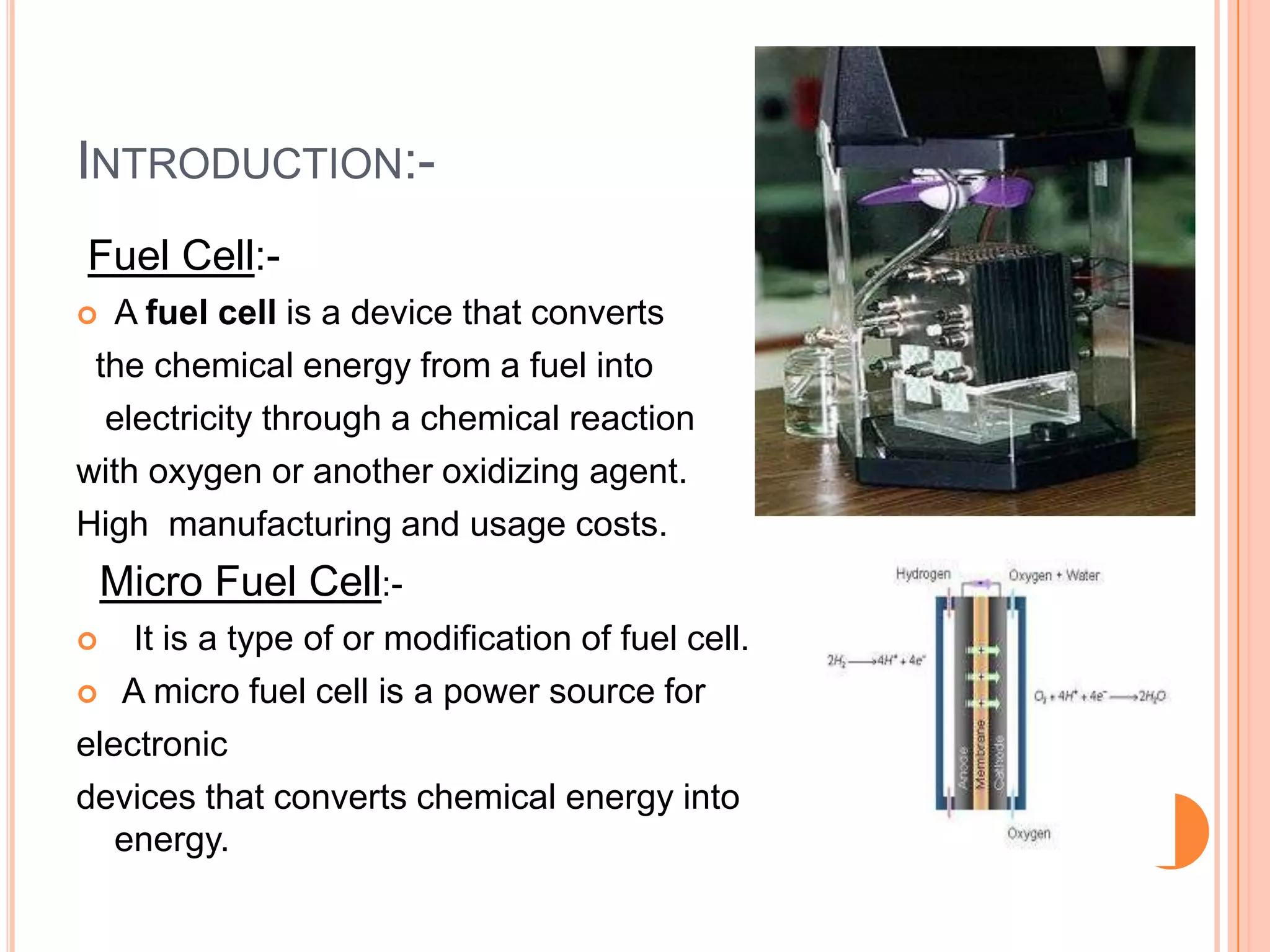
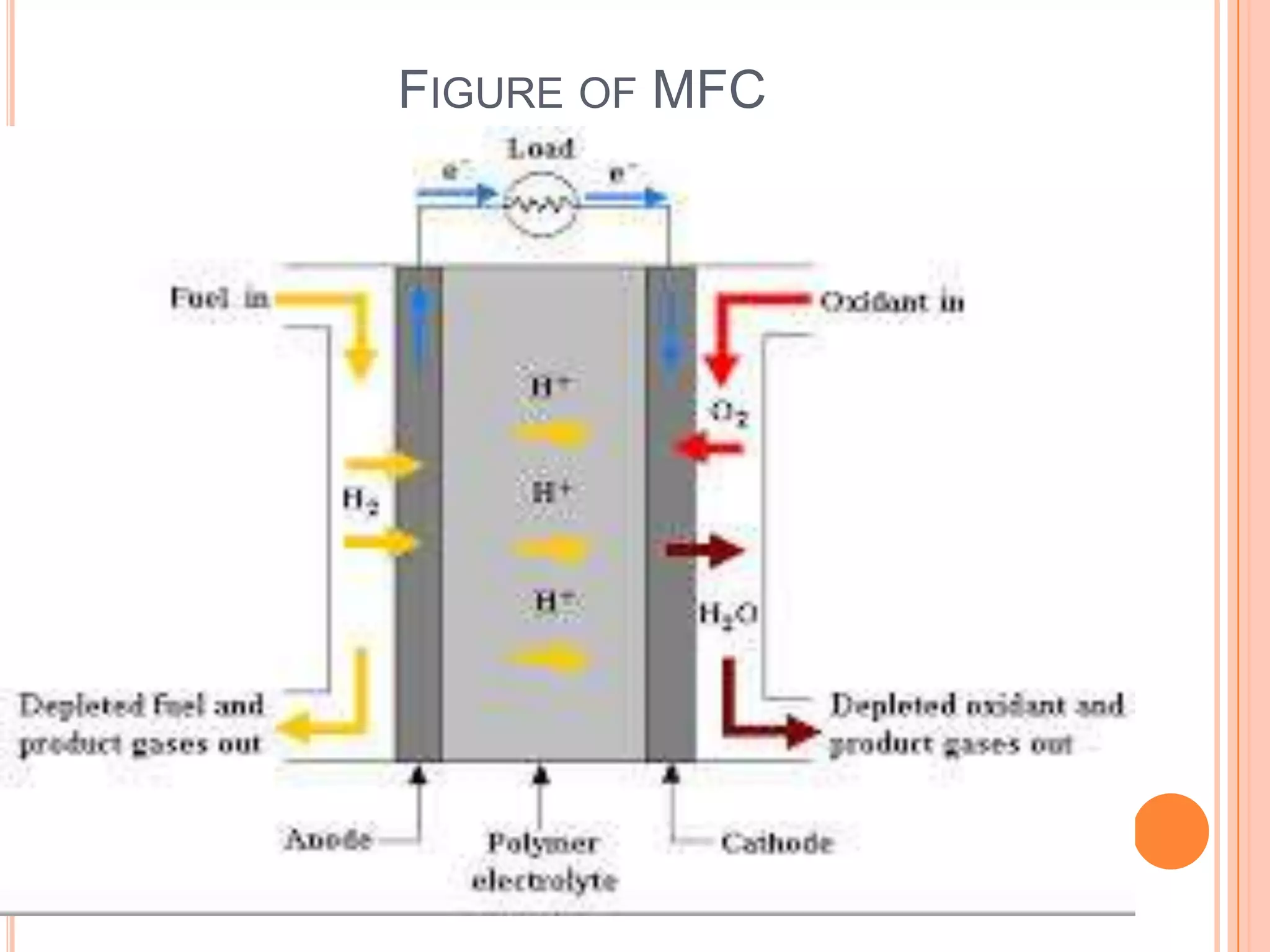
This seminar discusses micro fuel cells, which are a type of fuel cell that can power portable electronic devices. Micro fuel cells work by converting chemical energy directly from fuels like methanol into electrical energy through electrochemical reactions. They have several advantages over conventional batteries as they are reusable by refilling the fuel cartridge and do not need lengthy recharging times. The seminar covers the history, components, mechanisms, applications and future prospects of micro fuel cells.