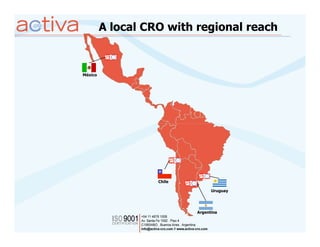
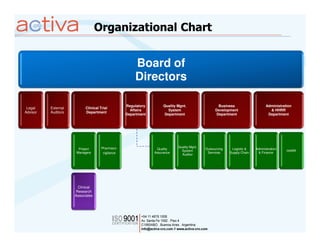
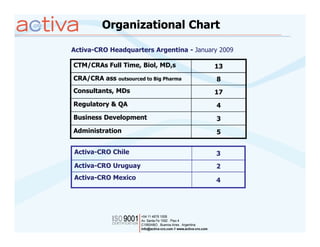
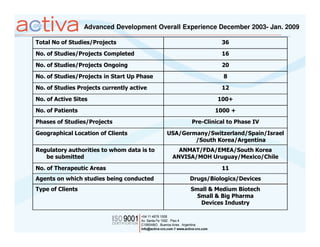
Activa is a full-service CRO based in Argentina established in 2003 with operations in Chile, Uruguay, and Mexico. It aims to be the leading regional Spanish-speaking CRO in Latin America through local expertise, training, and a quality assurance program. Activa offers clinical research, regulatory, pharmacovigilance, quality assurance, and training services across various therapeutic areas for small and large biotech and pharmaceutical clients.