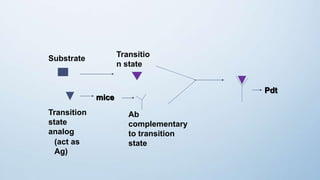
Abzymes, also known as catalytic antibodies, are monoclonal antibodies that exhibit enzymatic activity. They are able to bind to transition states of enzyme-catalyzed reactions with high specificity and affinity, stabilizing the transition state and increasing reaction rates. Abzymes can be artificially produced by immunizing animals with transition state analogs of reactions. They have potential applications in drug development, cancer treatment, and developing therapies for viral infections like HIV. Researchers have engineered an abzyme that can degrade an essential region of the HIV envelope protein, rendering the virus unable to infect cells.