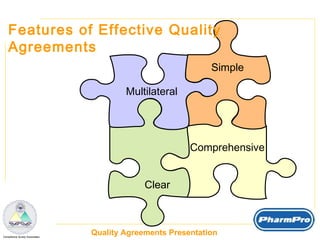
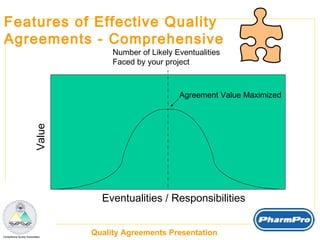
The document discusses quality agreements, which are documents that coordinate quality responsibilities between parties involved in pharmaceutical product development and manufacturing. Quality agreements help ensure compliance, manage risks, facilitate communication and planning, and define accountability. They should clearly establish responsibilities, materials handling and testing requirements, production and validation procedures, auditing standards, and other quality-related processes. Effective quality agreements are simple, clear, comprehensive, and have visibility throughout organizations.






















































![Ralph L. Dillon Director Compliance Surety Associates PO Box 908 Mundelein, IL 60060 USA Tel: 847-682-3924 E-mail: csa_c [email_address]](https://image.slidesharecdn.com/aapsqualityagreementpresentationshare-12491520824155-phpapp01/85/Aaps-Quality-Agreement-Presentation-Share-61-320.jpg)
![James P. McAndrew (Jim) Vice President PharmPro Services 2550 White Oak Circle Aurora, IL 60502-9678 USA Tel: 630-851-2550 E-mail: [email_address]](https://image.slidesharecdn.com/aapsqualityagreementpresentationshare-12491520824155-phpapp01/85/Aaps-Quality-Agreement-Presentation-Share-62-320.jpg)