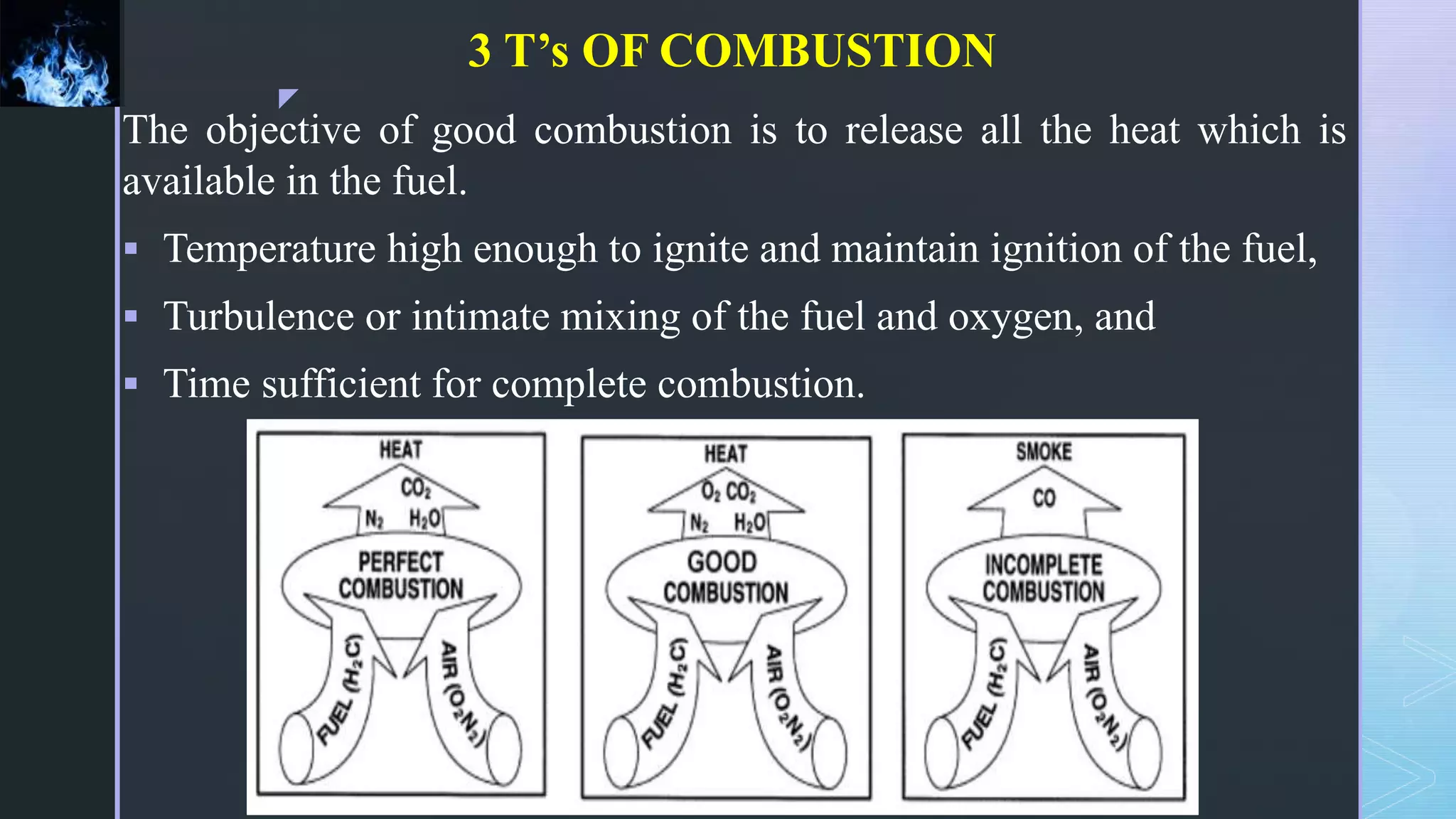
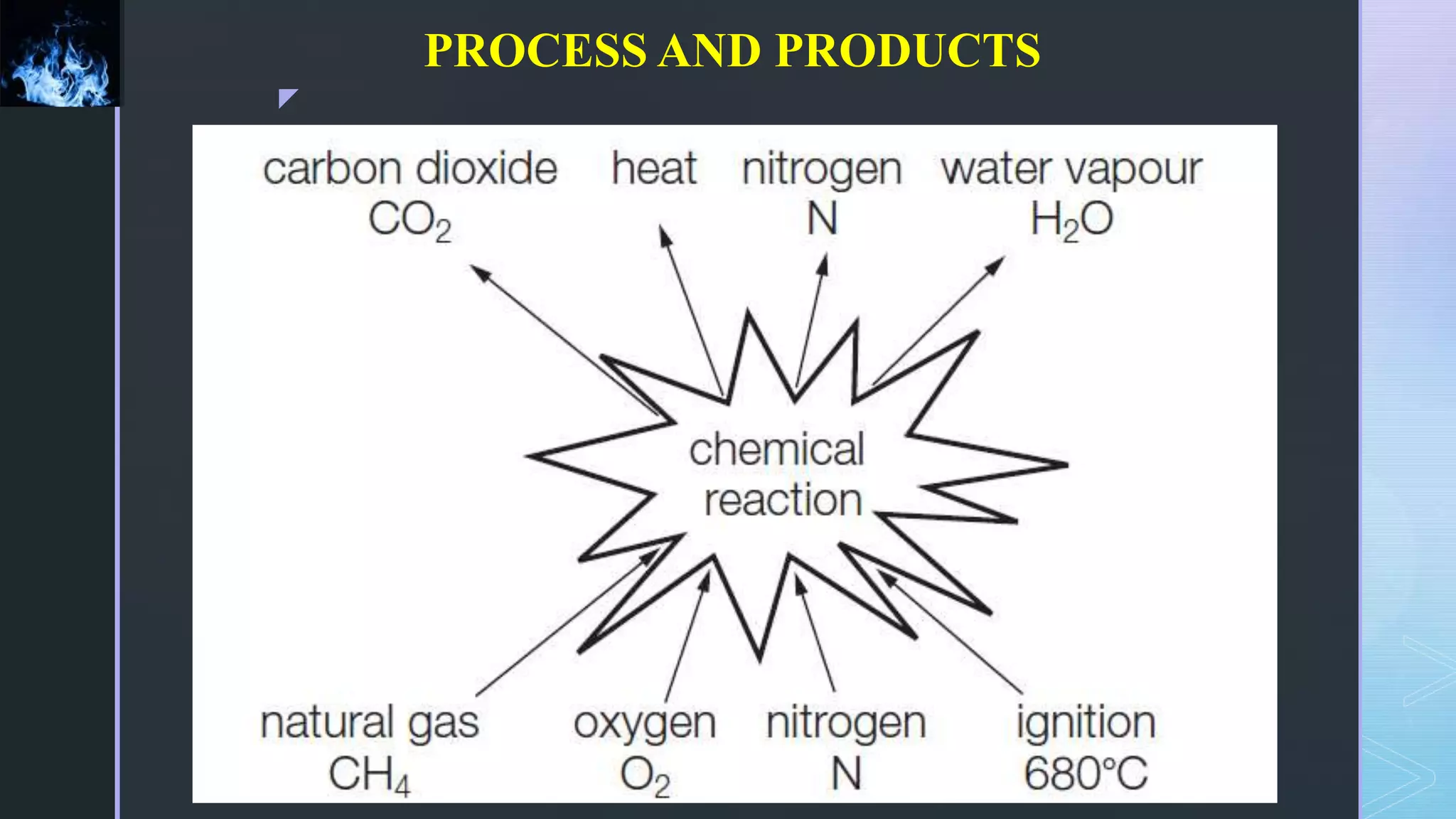
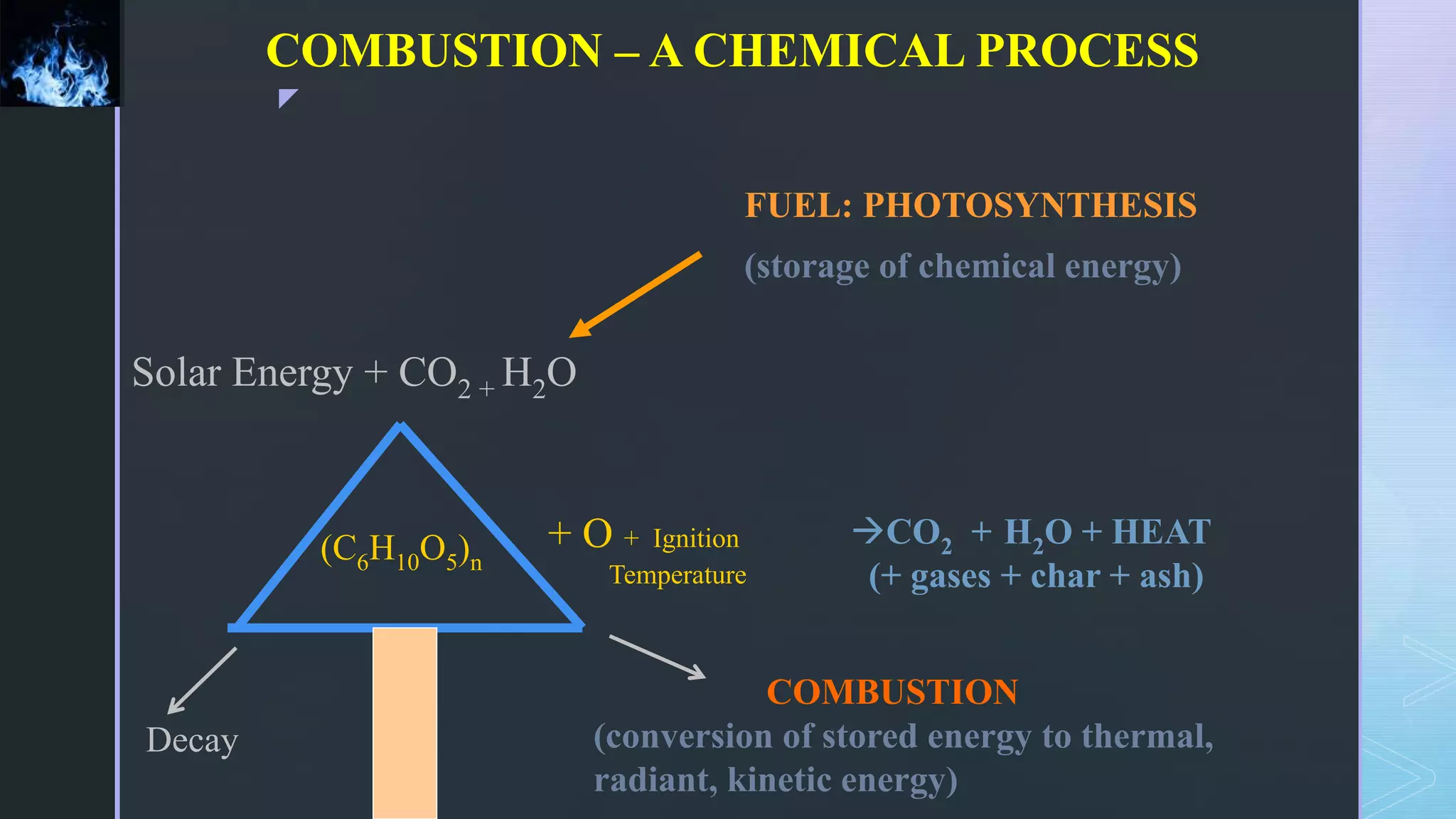
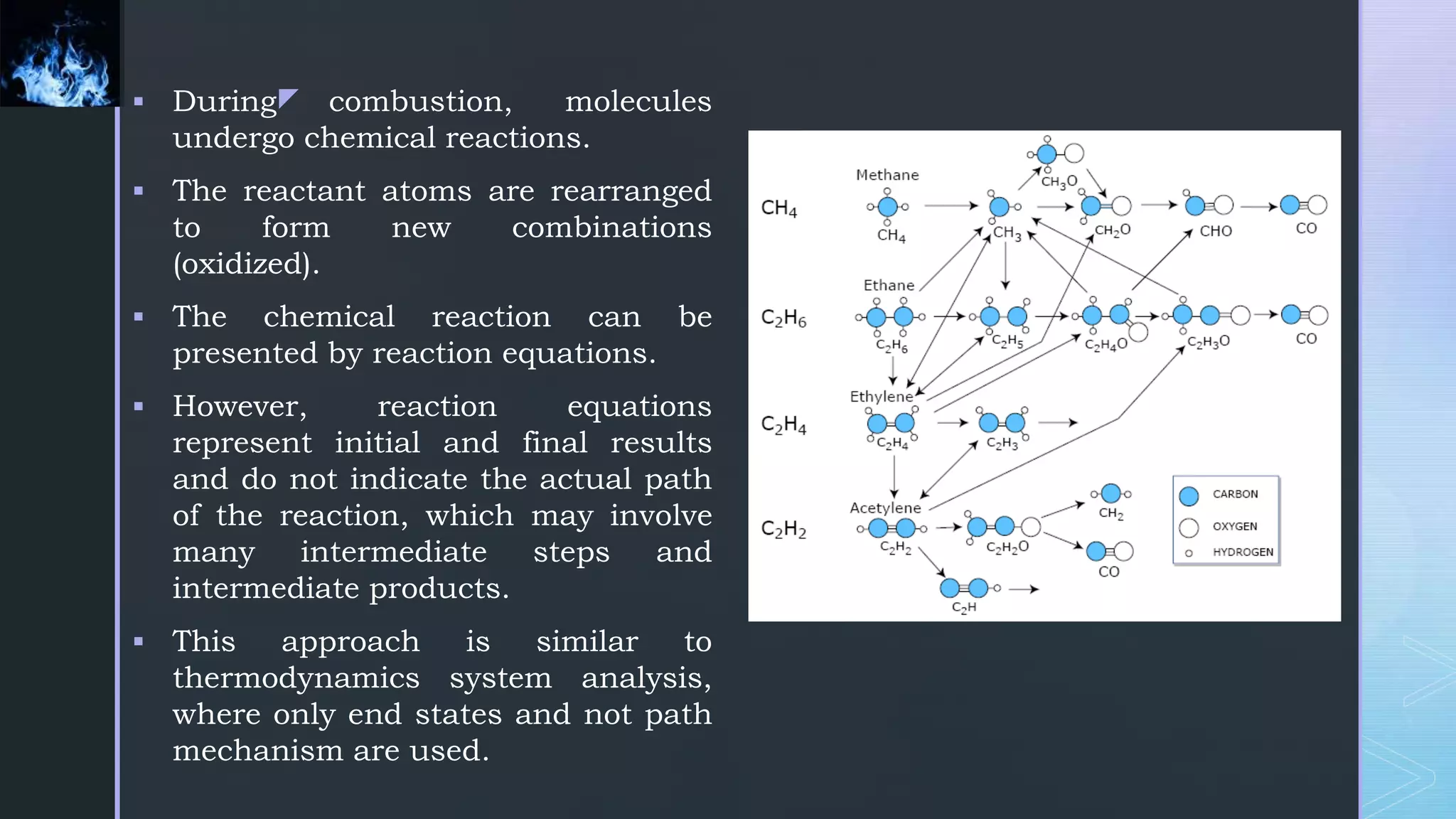
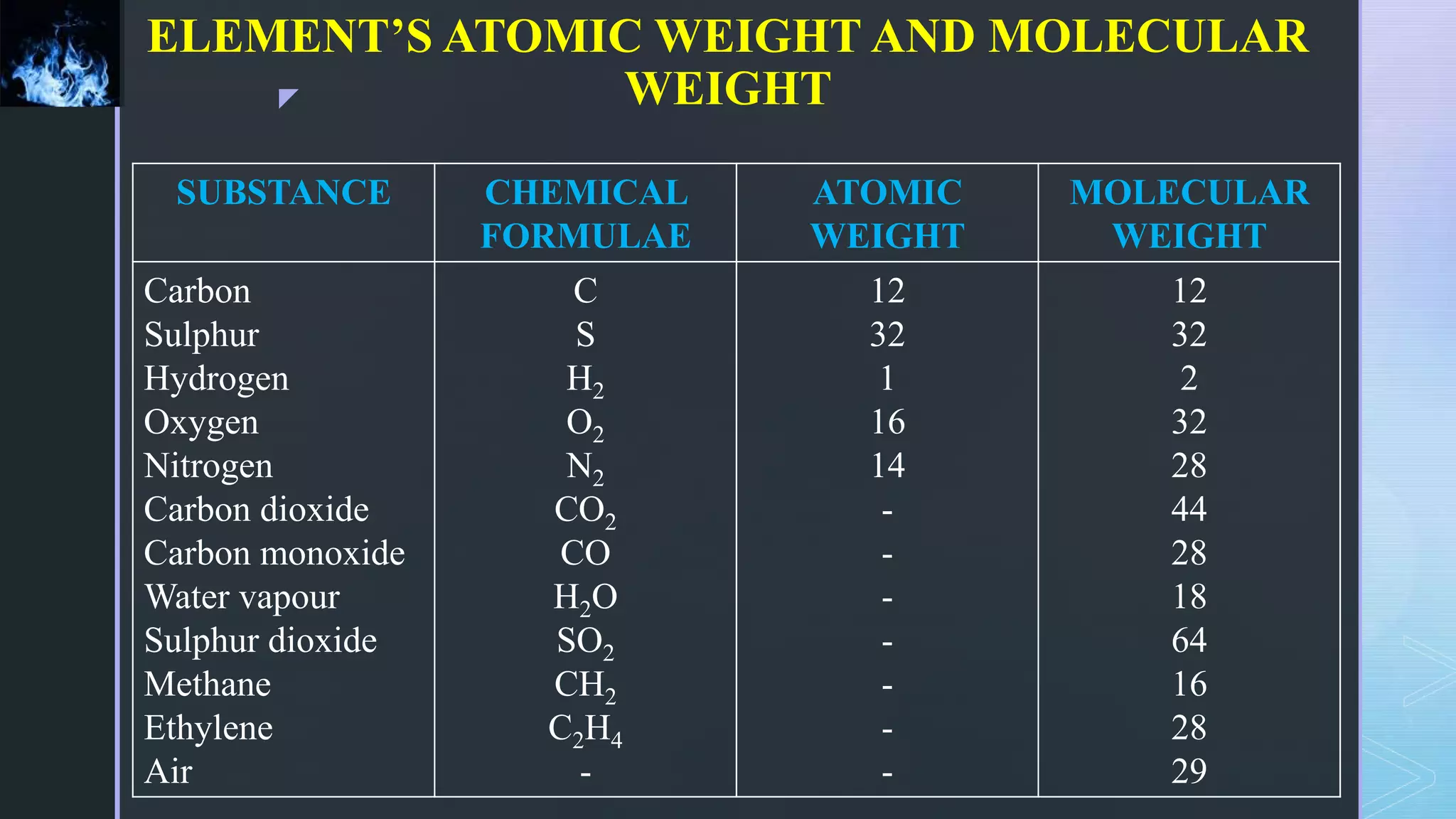
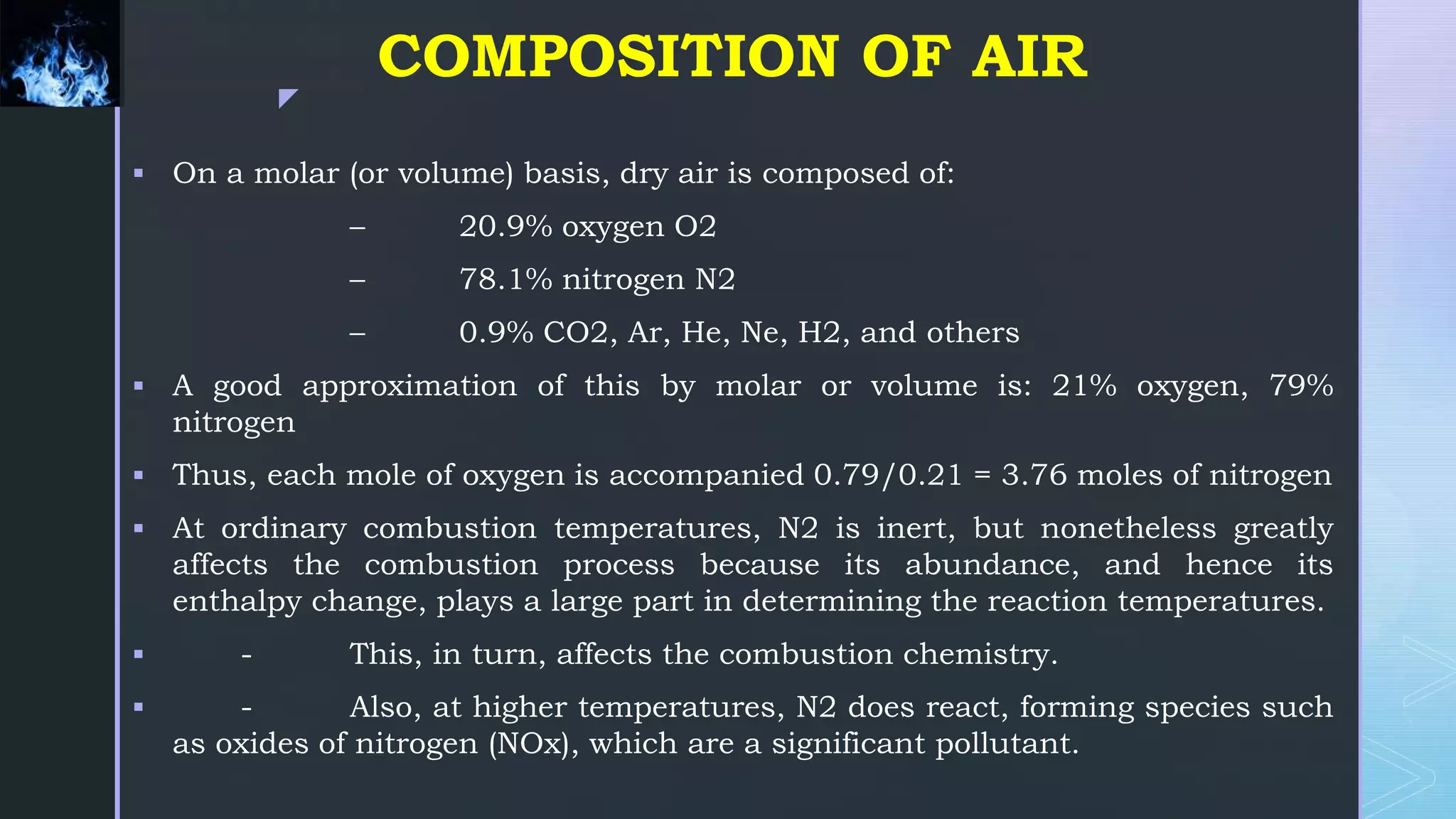
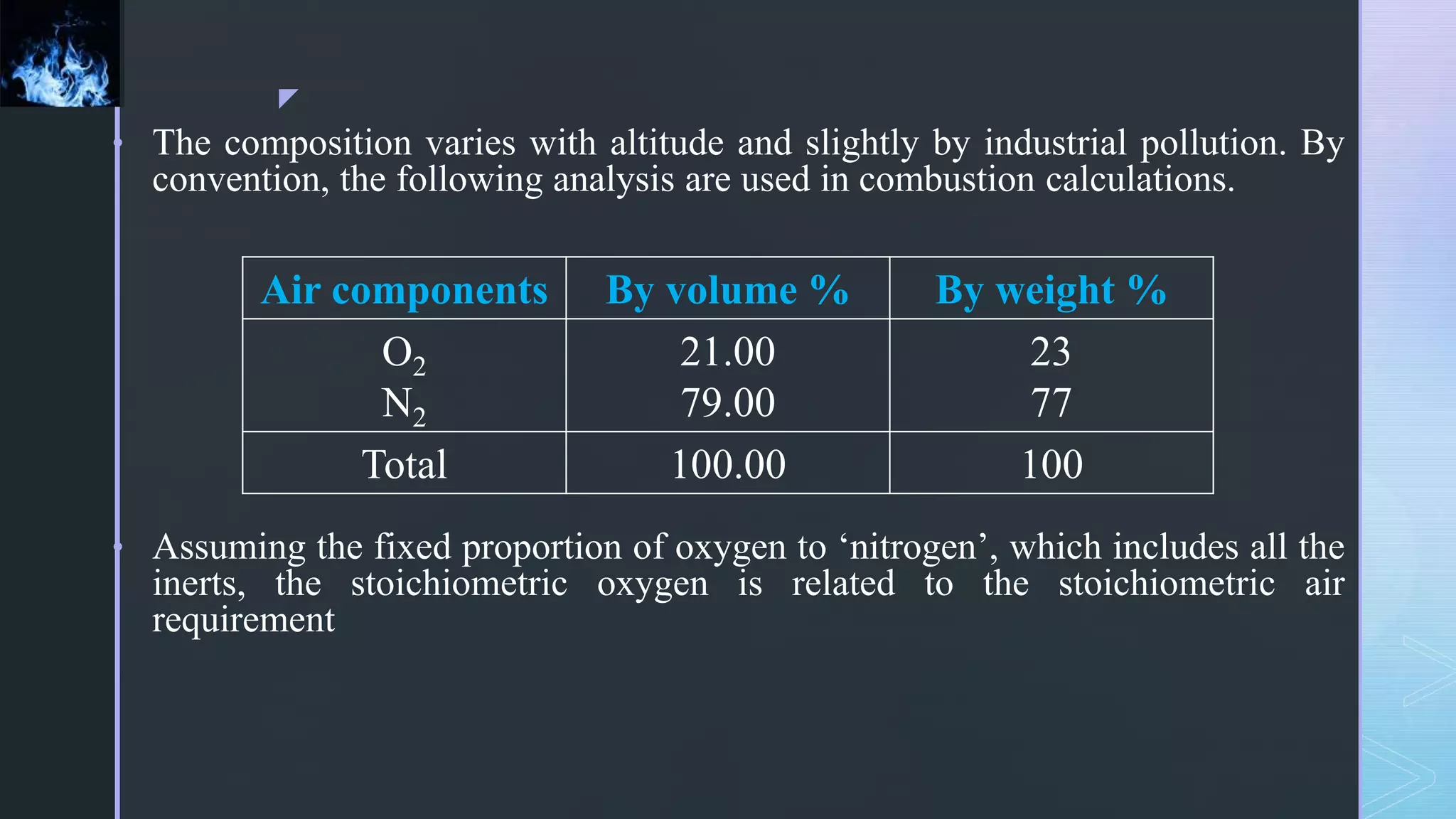
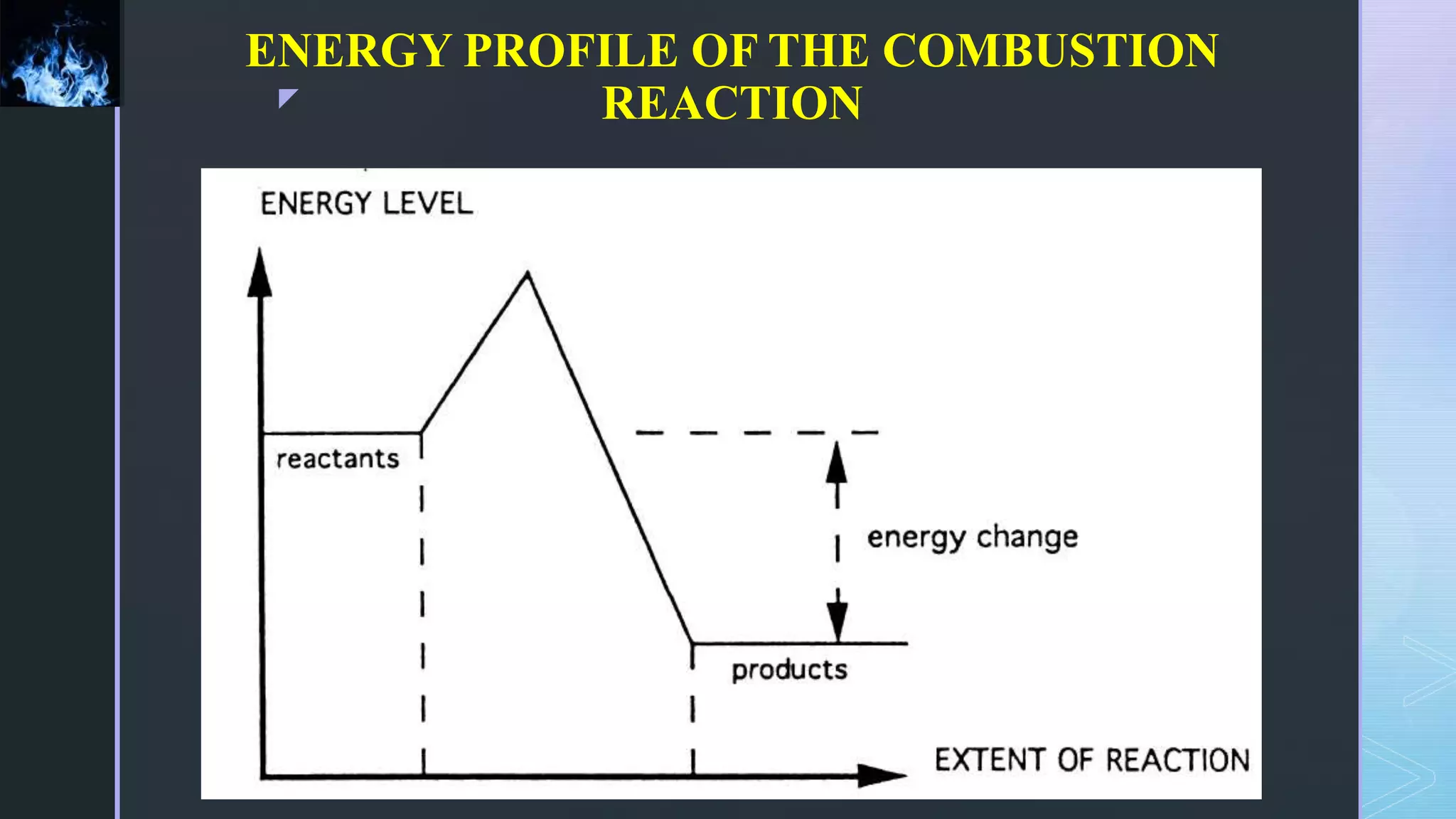
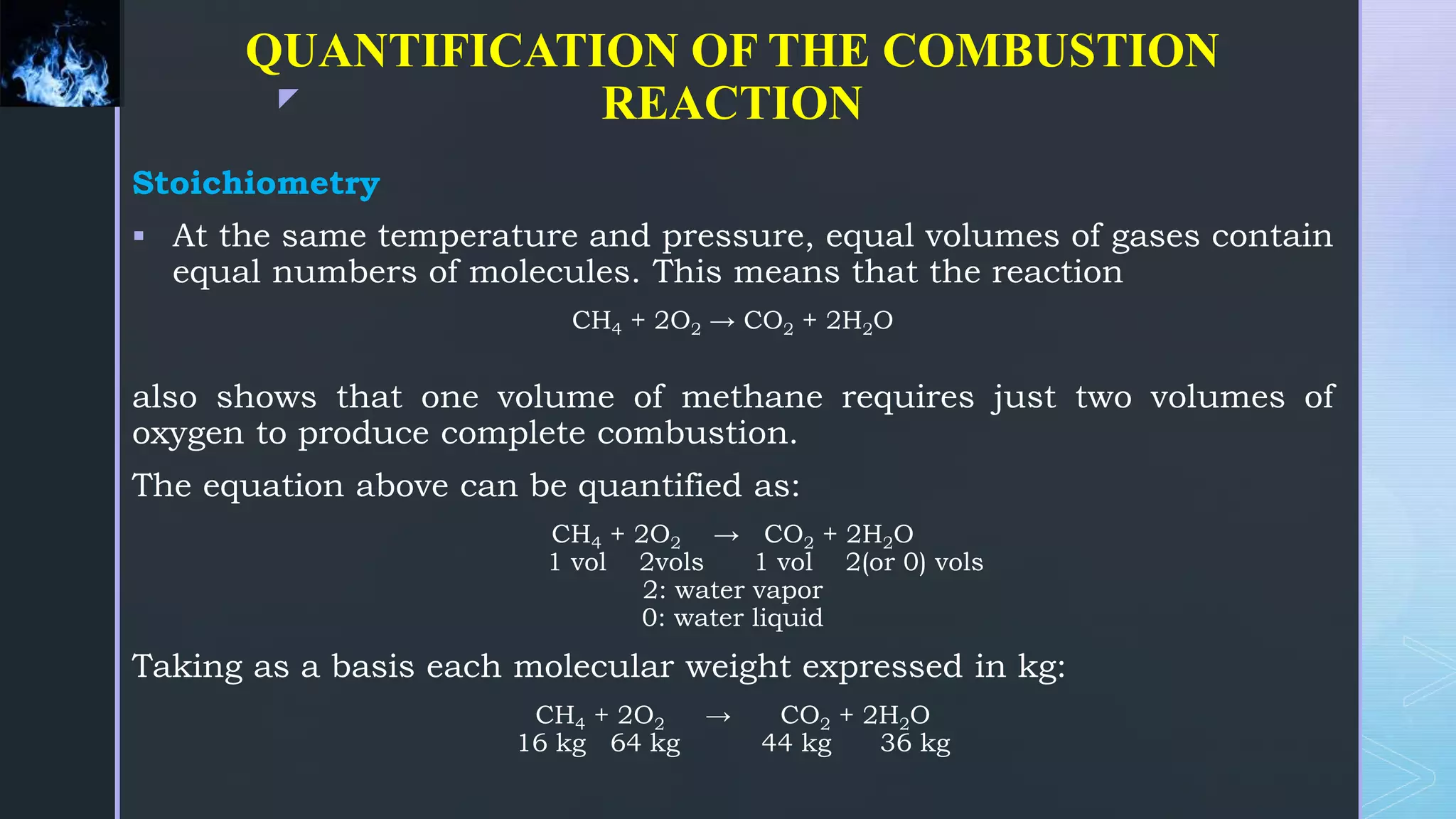
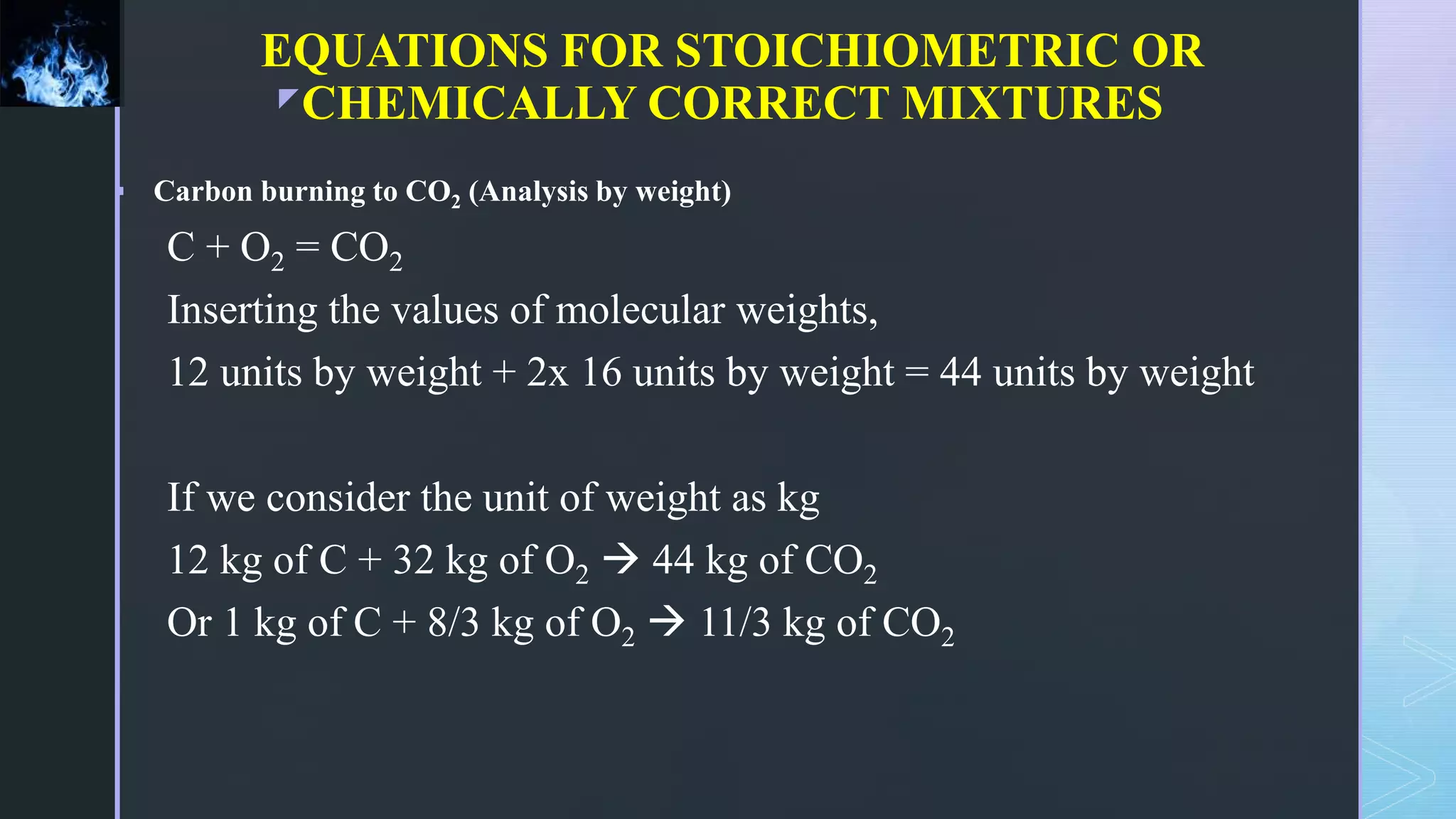
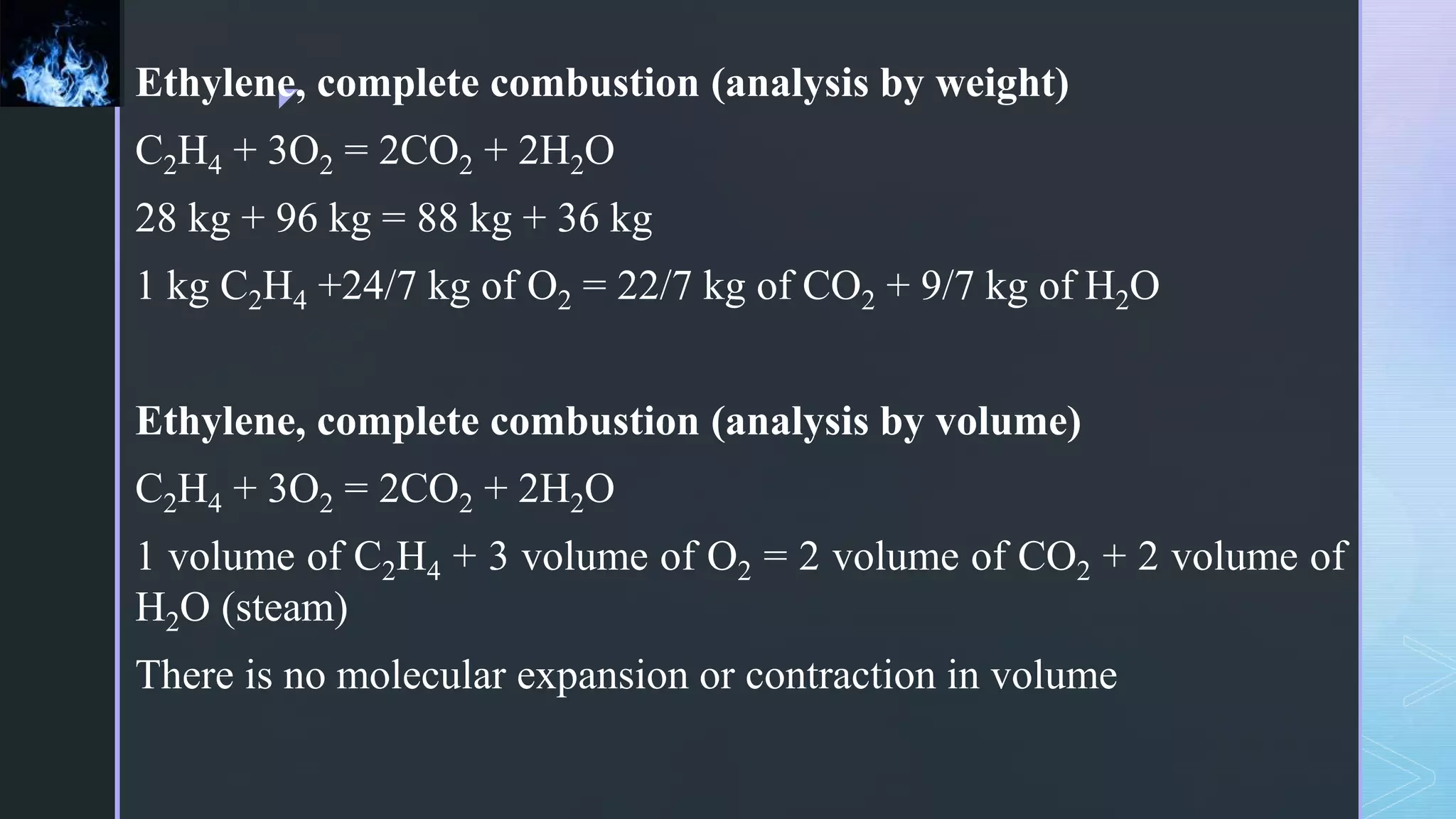
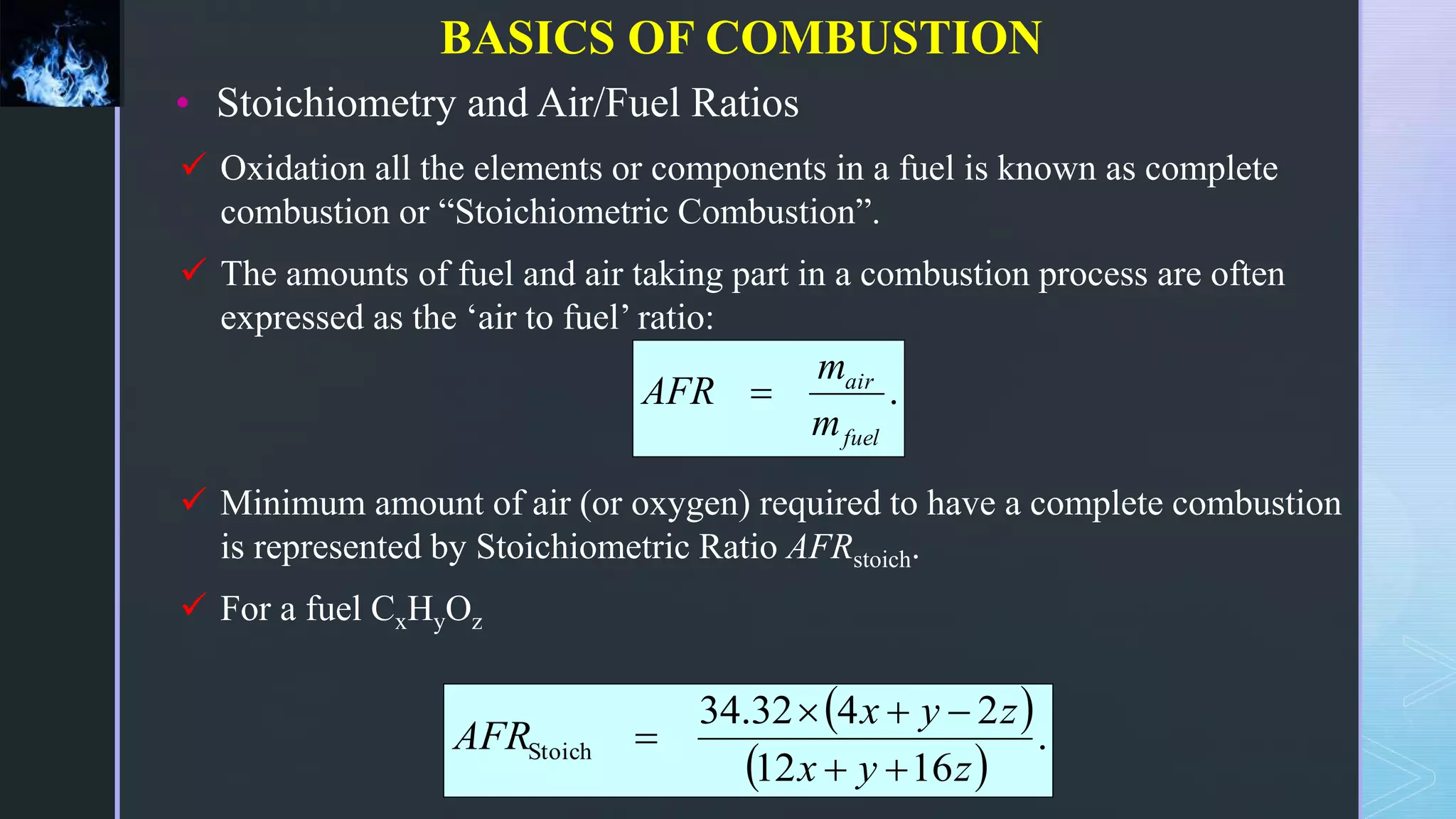
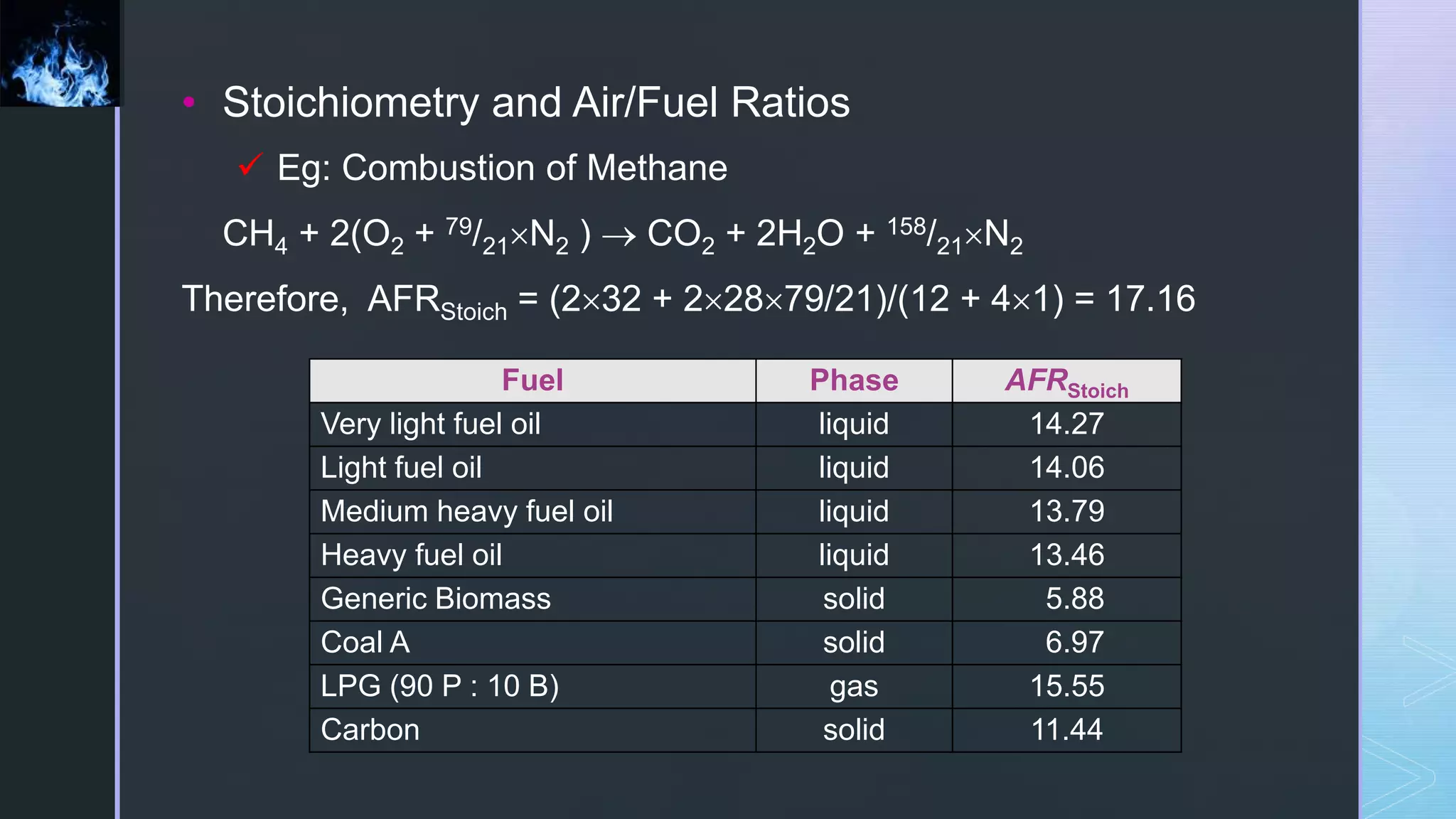
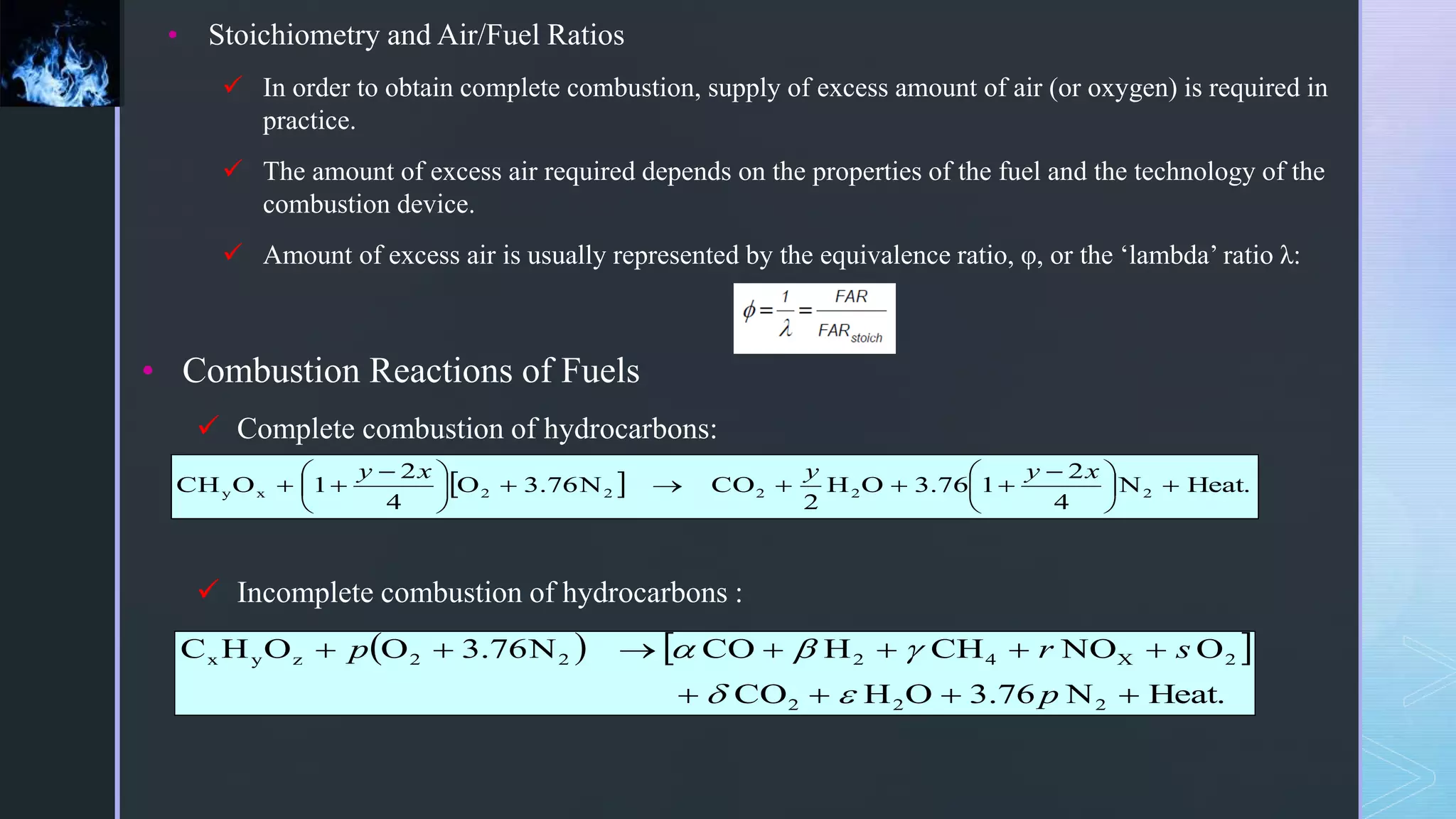
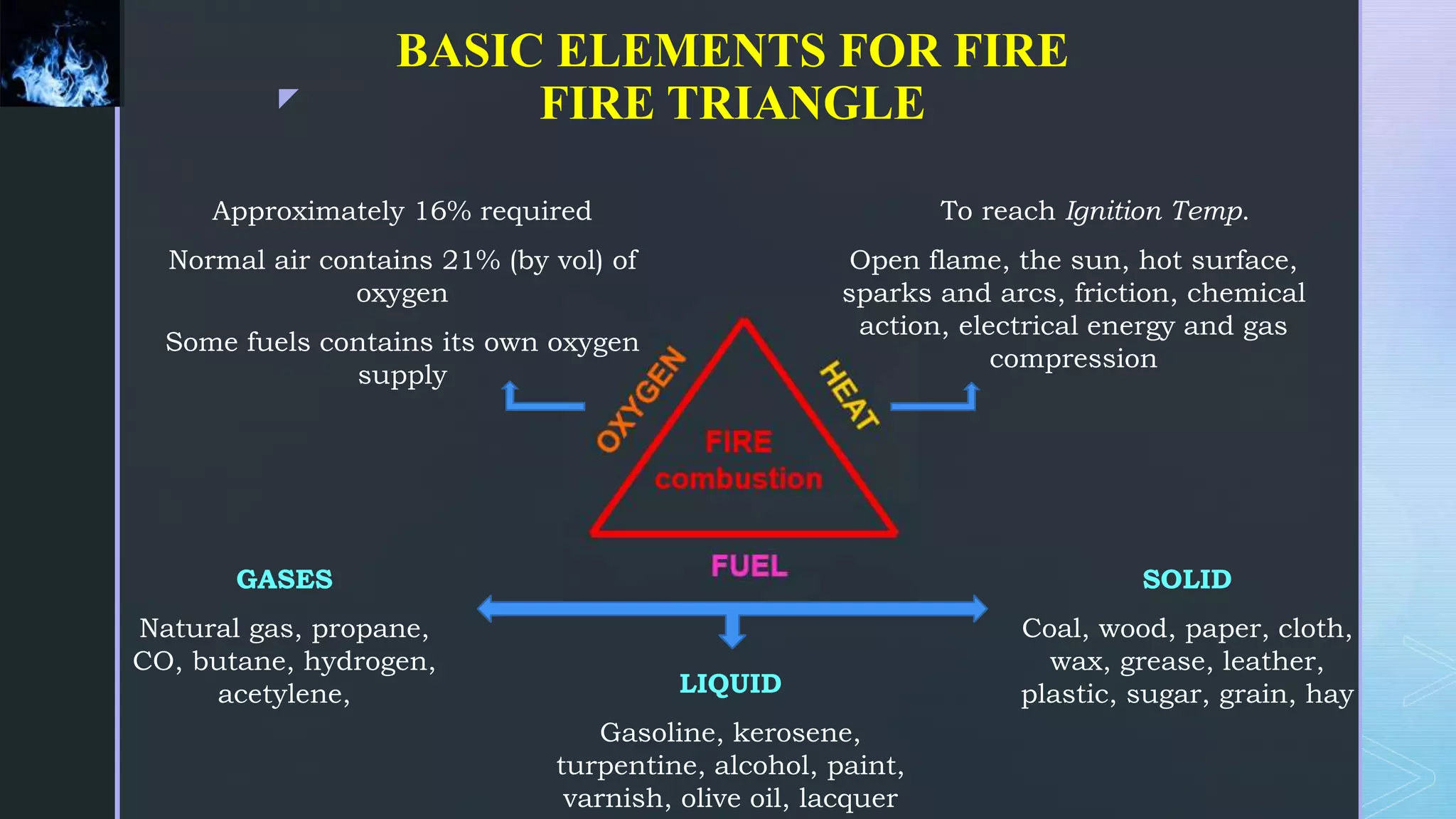
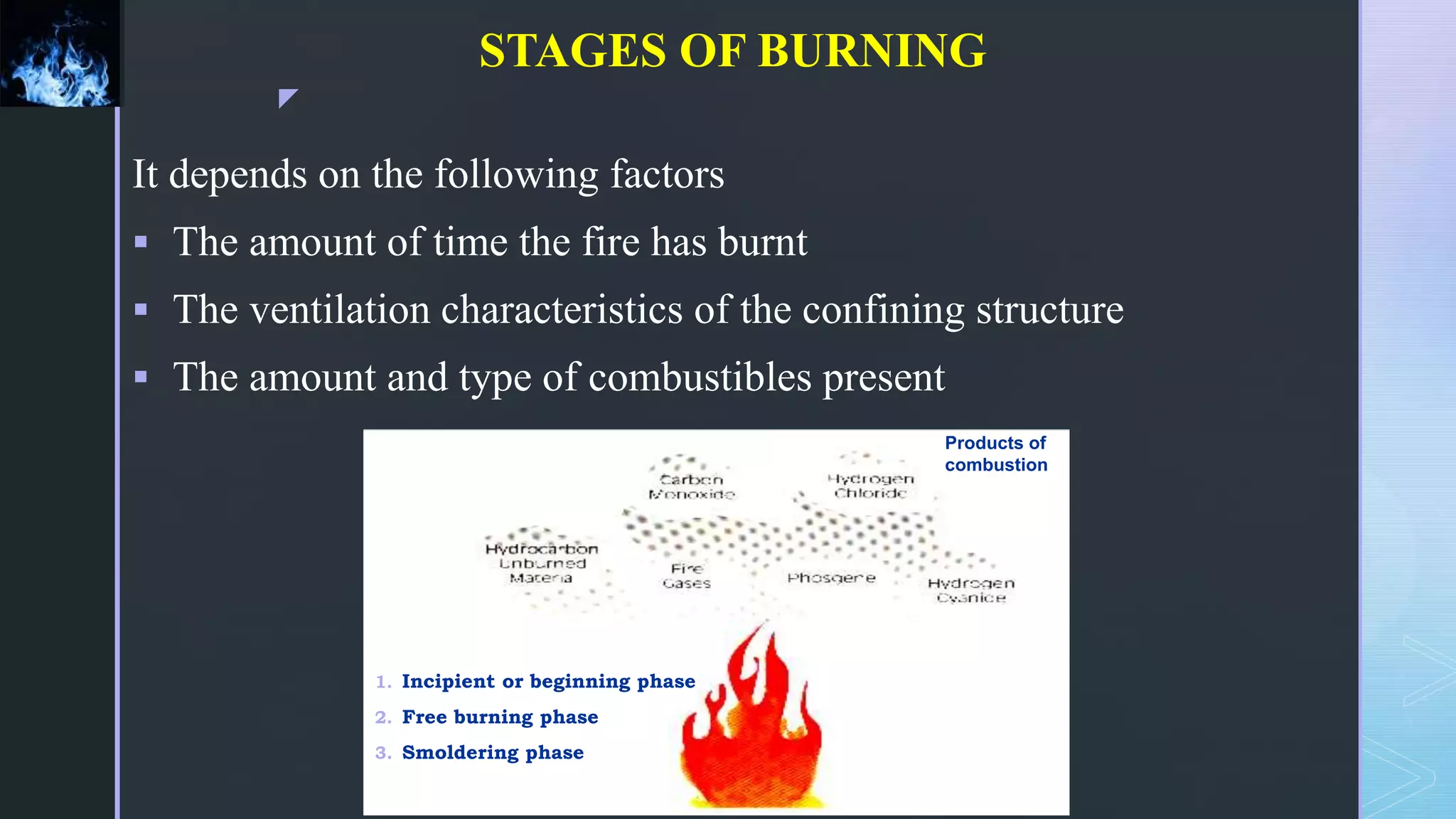
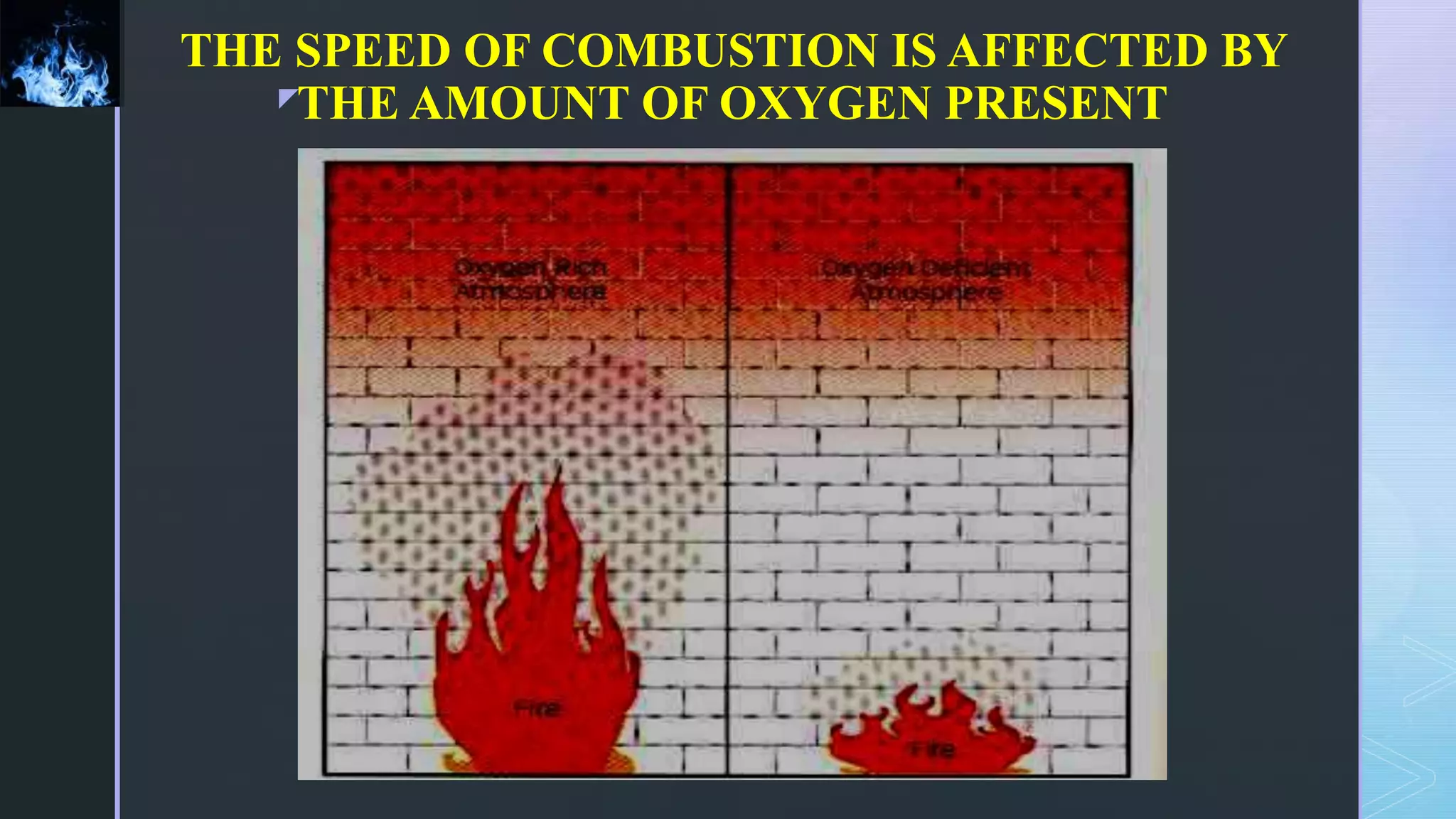
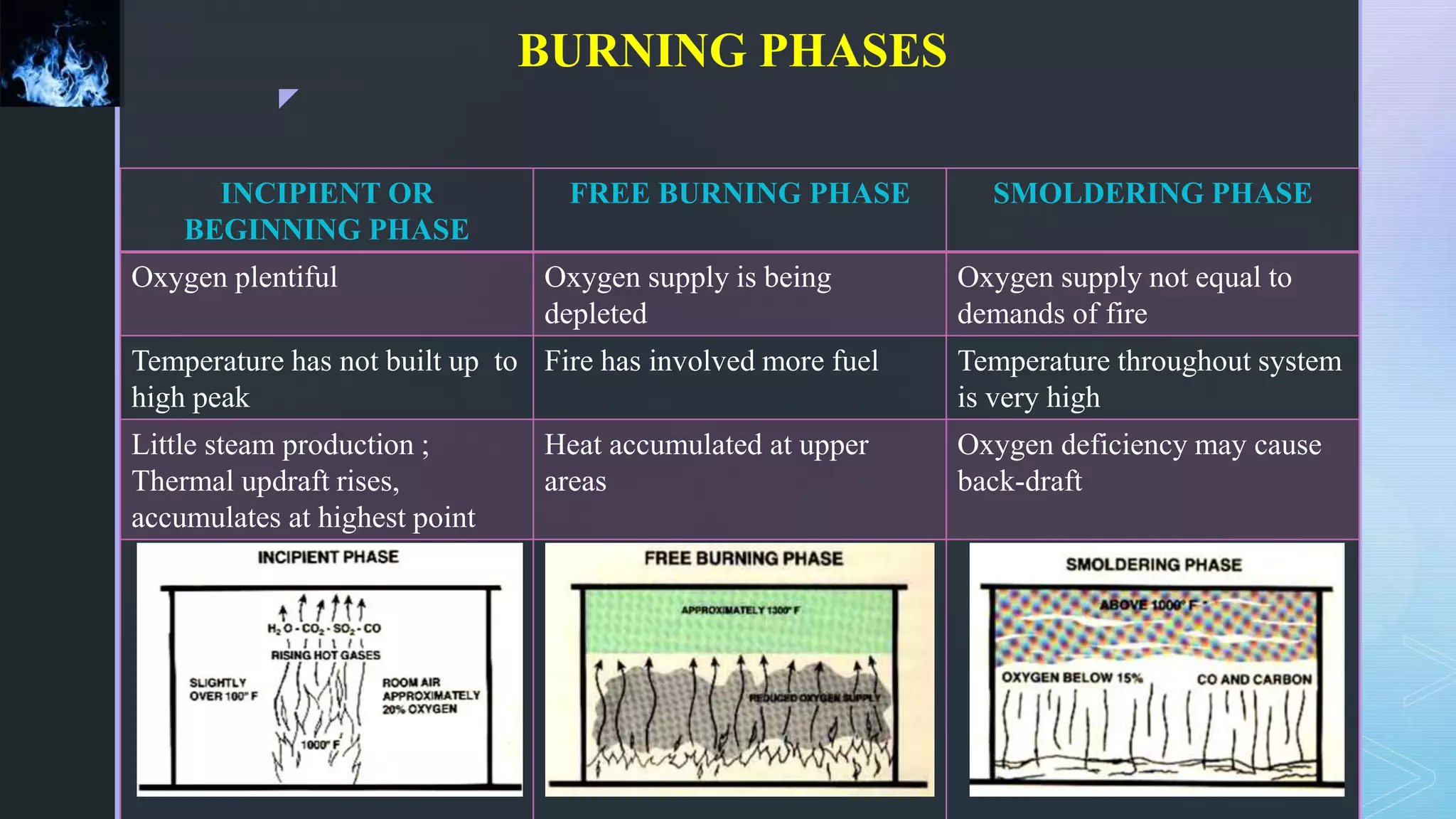
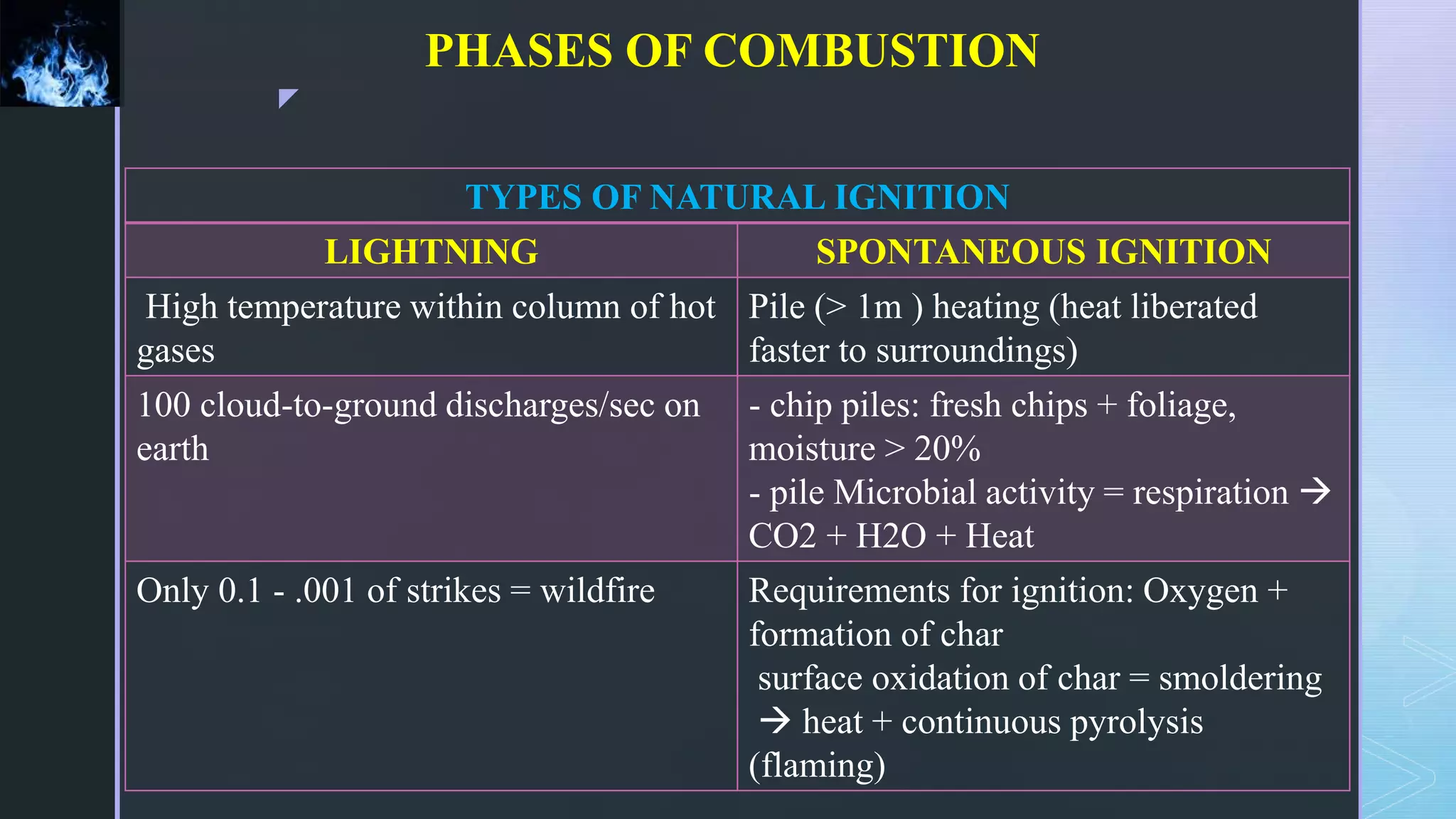
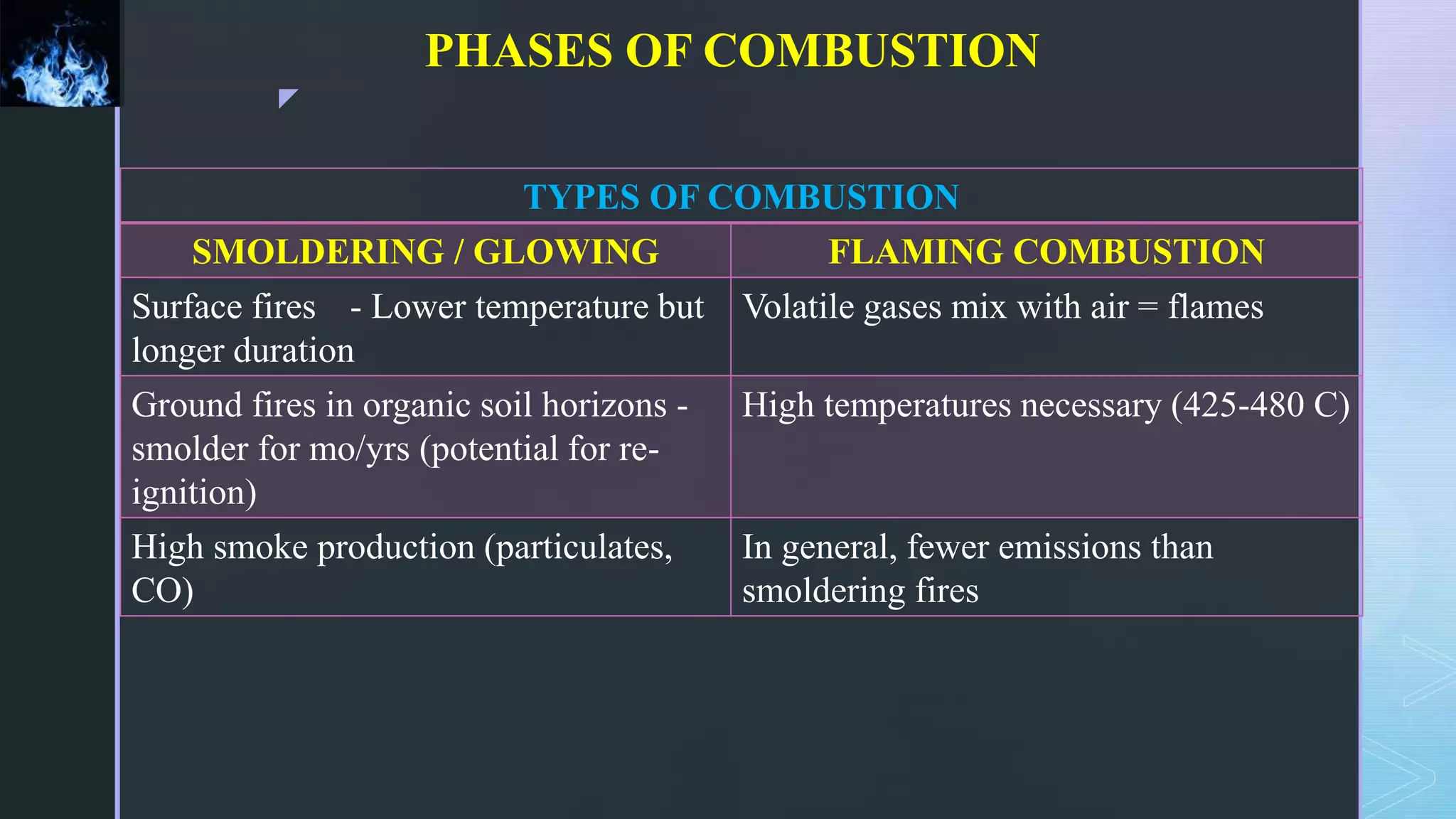
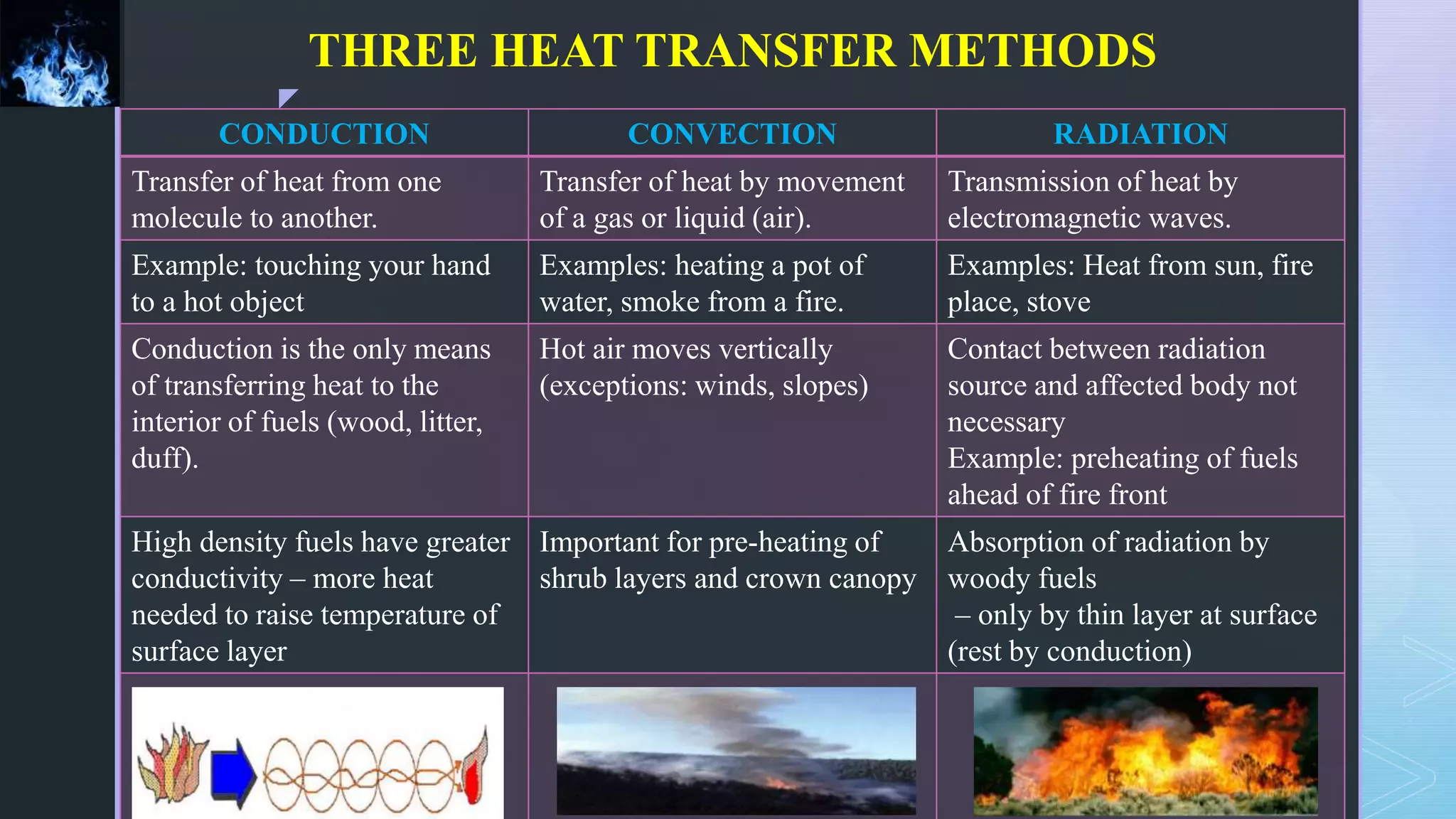
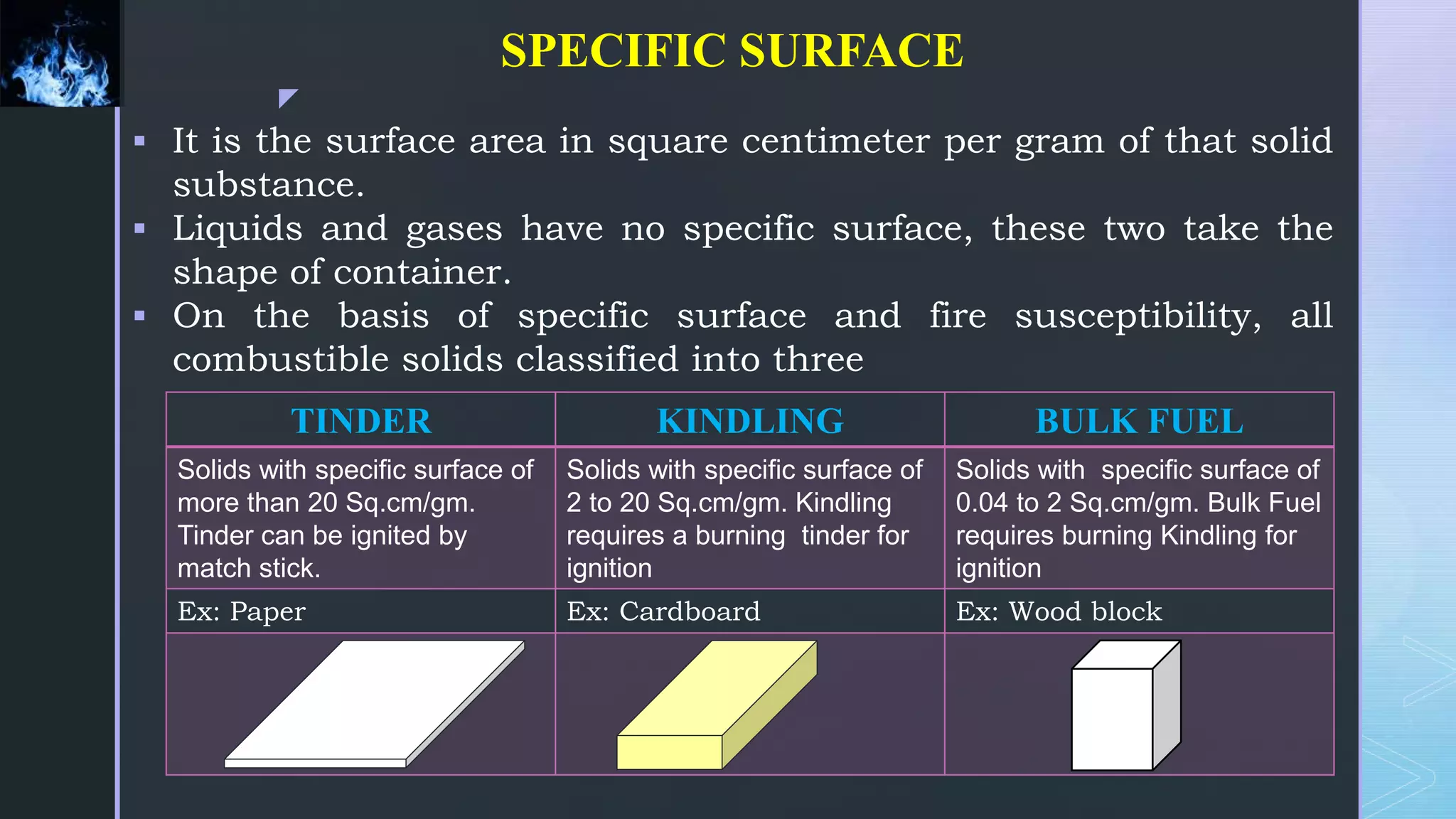
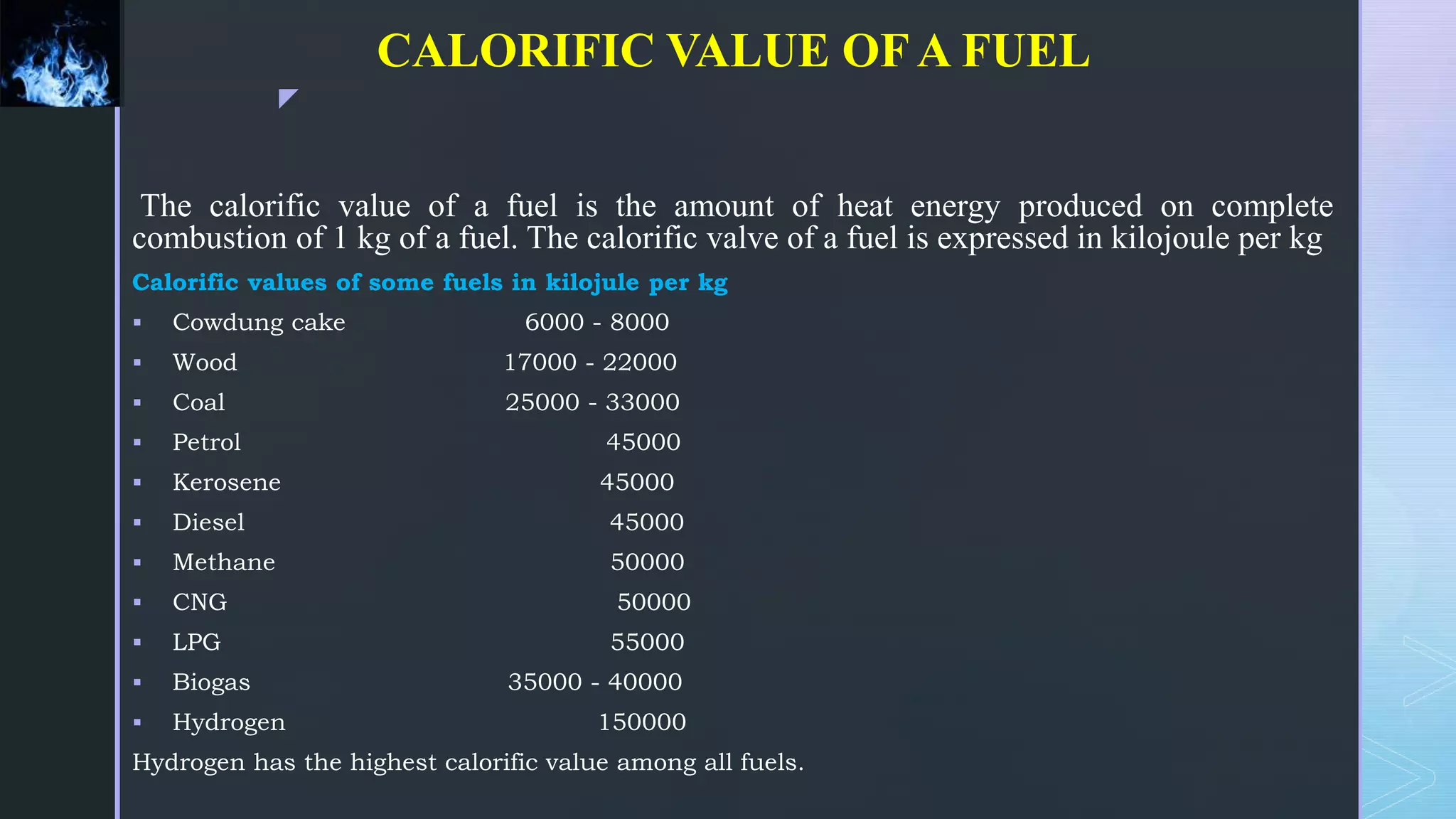
This document provides an overview of combustion principles, emphasizing the chemical reactions between fuel and oxygen that produce heat and light. It covers the processes involved in combustion, the importance of stoichiometry, and the roles of different fuels and oxidants, including air composition and the need for excess air for complete combustion. Additionally, it discusses the significance of complete and incomplete combustion, along with relevant equations and analysis methods for fuel combustion.