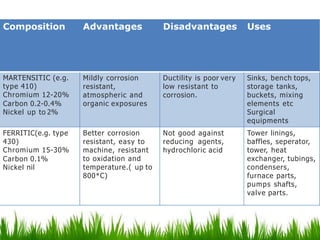
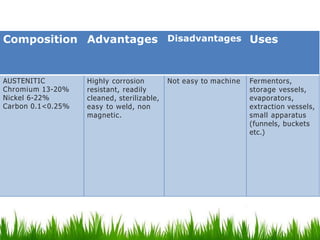
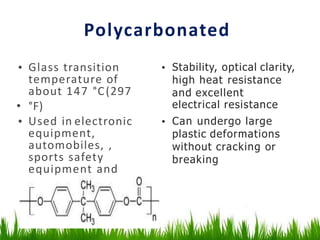
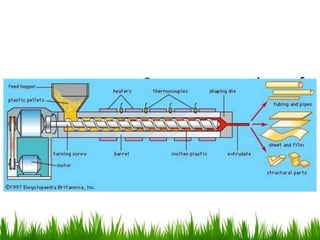
This document discusses materials used in pharmaceutical manufacturing plant equipment. It covers factors that affect material selection such as physical and chemical properties as well as cost. Various metal materials are described including ferrous metals like cast iron, carbon steel, and stainless steel, as well as non-ferrous metals like aluminum, copper, nickel, and titanium. Non-metal materials discussed include glass, rubber, plastics like PVC, polyethylene, and polypropylene. Each material's properties, advantages, disadvantages and applications are summarized.