This document contains instructions for a test booklet and answer sheet. It provides details like the number of pages in the test booklet, duration of the exam, number of questions, distribution of questions across subjects, marking scheme, and important instructions for candidates regarding filling the answer sheet and guidelines during the exam. The document also mentions the test booklet code.


















![E/Page 20 SPACE FOR ROUGH WORK / ÚUȤ ·¤æØü ·ð¤ çÜ° Á»ã
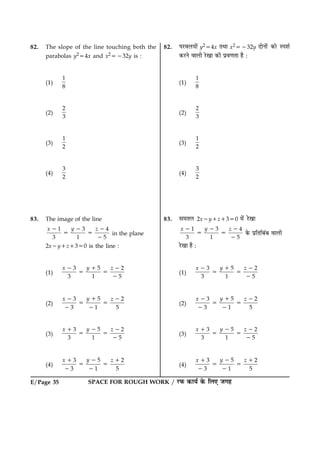
40. For the non - stoichiometre reaction
2A1B ® C1D, the following kinetic data
were obtained in three separate
experiments, all at 298 K.
Initial
Concentration
(A)
Initial
Concentration
(B)
Initial rate of
formation of C
(mol L2
S2
)
0.1 M 0.1 M 1.2 3 1023
0.1 M 0.2 M 1.2 3 1023
0.2 M 0.1 M 2.4 3 1023
The rate law for the formation of C is :
(1)
dc
dt
5k[A] [B]
(2)
dc
dt
5k[A]2 [B]
(3)
dc
dt
5k[A] [B]2
(4)
dc
dt
5k[A]
41. Among the following oxoacids, the correct
decreasing order of acid strength is :
(1) HOCl > HClO2 > HClO3 > HClO4
(2) HClO4 > HOCl > HClO2 > HClO3
(3) HClO4 > HClO3 > HClO2 > HOCl
(4) HClO2 > HClO4 > HClO3 > HOCl
42. The metal that cannot be obtained by
electrolysis of an aqueous solution of its
salts is :
(1) Ag
(2) Ca
(3) Cu
(4) Cr
40. ÚUâæØçÙ·¤Ìæ çÚU€Ì ¥çÖç·ý¤Øæ 2A1B ® C1D ×ð´
ÌèÙ ÂëÍ·¤ ÂýØæð»æð´ ×ð´ 298 K ÂÚU çÙÙ »çÌ·¤ ¥æ´·¤Ç¸ð
ÂýæŒÌ ç·¤Øð »Øð Ñ
§âË¿Ũ»œ‰
ÇË™³âøË (A)
§âË¿Ũ»œ‰
ÇË™³âøË (B)
C º¾¾Õ œ‰Í §âË¿Ũ»œ‰
³¿U (¼ËÕÁ L2
S2
)
0.1 M 0.1 M 1.2 3 1023
0.1 M 0.2 M 1.2 3 1023
0.2 M 0.1 M 2.4 3 1023
¥çÖç·ý¤Øæ ·ð¤ çÜØð C ÕÙÙð ·¤æ ÎÚU çÙØ× ãæð»æ Ñ
(1)
dc
dt
5k[A] [B]
(2)
dc
dt
5k[A]2 [B]
(3)
dc
dt
5k[A] [B]2
(4)
dc
dt
5k[A]
41. çÙÙ ¥æ€âæð ¥Üæð´ ·ð¤ çÜØð ¥Ü àæç€Ì ·¤æ ØÍæÍü
ƒæÅUÌæ ·ý¤× ãæð»æ Ñ
(1) HOCl > HClO2 > HClO3 > HClO4
(2) HClO4 > HOCl > HClO2 > HClO3
(3) HClO4 > HClO3 > HClO2 > HOCl
(4) HClO2 > HClO4 > HClO3 > HOCl
42. ÏæÌé Áæð ¥ÂÙð Ü߇ææð´ ·ð¤ ÁÜèØ çßÜØÙæð´ ·ð¤
§Üñ€ÅþUæÜðçââ (çßléÌ ¥ÂƒæÅUÙ) âð ÂýæŒÌ Ùãè´ ãæð
â·¤Ìè ãæðÌè ãñ Ñ
(1) Ag
(2) Ca
(3) Cu
(4) Cr](https://image.slidesharecdn.com/06-150313233700-conversion-gate01/85/06-04-2014-e-20-320.jpg)


![SPACE FOR ROUGH WORK / ÚUȤ ·¤æØü ·ð¤ çÜ° Á»ãE/Page 23
49. Which series of reactions correctly
represents chemical relations related to
iron and its compound ?
(1) 2 4 2 4 2dil H SO H SO , O
4Fe FeSO£££££“ £££££“
heat
2 4 3Fe (SO ) Fe£££“
(2) 2 2 4O , heat dil H SO
Fe FeO££££“ £££££“
heat
4FeSO Fe£££“
(3) 2Cl , heat heat, air
3Fe FeCl£££££“ ££££“
Zn
2FeCl Fe££“
(4) 2O , heat CO, 600 C
3 4Fe Fe O££££“ £££££“
8
CO, 700 C
FeO Fe£££££“
8
50. The equation which is balanced and
represents the correct product(s) is :
(1) Li2O12KCl ® 2LiCl1K2O
(2) [CoCl(NH3)5]115H1®Co21
15 4NH1
1Cl2
(3) [ M g ( H 2 O ) 6 ] 2 1 1 ( E D T A ) 4 2
excess NaOH
££££££“ [ M g ( E D T A ) ] 2 1
1 6H2O
(4) CuSO414KCN®K2[Cu(CN)4]
1K2SO4
49. §Ù×ð´ âð ¥çÖç·ý¤Øæ¥æð´ ·¤æ ·¤æñÙ-âæ ·ý¤× ØÍæÍü M¤Â ×ð´
Üæðãð ¥æñÚU §â·ð¤ Øæñç»·¤æð´ ·¤è ÚUæâæØçÙ·¤ ¥çÖç·ý¤Øæ¥æð´
·¤æð çÙM¤çÂÌ ·¤ÚUÌæ ãñ?
(1) 2 4 2 4 2H SO H SO , O
4Fe FeSO£££££“ £££££“
±¾Î
2 4 3Fe (SO ) Fe£££“
±Ë§
(2) 2 2 4O , H SO
Fe FeO££££“ £££££“
±Ë§ ±¾Î
4FeSO Fe£££“
±Ë§
(3) 2Cl ,
3Fe FeCl££££“ ££££“
±Ë§ ±Ë§, Ä˽Î
Zn
2FeCl Fe££“
(4) 2O , CO, 600 C
3 4Fe Fe O££££“ £££££“
8±Ë§
CO, 700 C
FeO Fe£££££“
8
50. â×è·¤ÚU‡æ Áæð â´ÌéçÜÌ ãñ ¥æñÚU ØÍæÍü ç·ý¤Øæ ȤÜæð´ ·¤è
âê¿·¤ ãñ, ãñ Ñ
(1) Li2O12KCl ® 2LiCl1K2O
(2) [CoCl(NH3)5]115H1®Co21
15 4NH1
1Cl2
(3) [ M g ( H 2 O ) 6 ] 2 1 1 ( E D T A ) 4 2
NaOH
£££££££“
œ‰Ë ŠËÌ´þ½ [Mg(EDTA)]21
1 6H2O
(4) CuSO414KCN®K2[Cu(CN)4]
1K2SO4](https://image.slidesharecdn.com/06-150313233700-conversion-gate01/85/06-04-2014-e-23-320.jpg)




![E/Page 28 SPACE FOR ROUGH WORK / ÚUȤ ·¤æØü ·ð¤ çÜ° Á»ã
PART C — MATHEMATICS Öæ» C — »ç‡æÌ
61. If X5{4n23n21 : n e N} and
Y5{9(n21) : n e N}, where N is the set of
natural numbers, then XÈY is equal to :
(1) X
(2) Y
(3) N
(4) Y2X
62. If z is a complex number such that ?z?/2,
then the minimum value of
1
z
2
1 :
(1) is strictly greater than
5
2
(2) is strictly greater than
3
2
but less
than
5
2
(3) is equal to
5
2
(4) lies in the interval (1, 2)
63. If a e R and the equation
23(x2[x])212 (x2[x])1a250
(where [x] denotes the greatest integer
[ x) has no integral solution, then all
possible values of a lie in the interval :
(1) (22, 21)
(2) (2:, 22) È (2, :)
(3) (21, 0) È (0, 1)
(4) (1, 2)
61. ØçÎ X5{4n23n21 : n e N} ÌÍæ
Y5{9(n21) : n e N} ãñ´, Áãæ¡ N, Âýæ·ë¤Ì â´Øæ¥æð´
·¤æ â×é“æØ ãñ, Ìæð XÈY ÕÚUæÕÚU ãñ Ñ
(1) X
(2) Y
(3) N
(4) Y2X
62. ØçÎ z °·¤ °ðâè âçןæ â´Øæ ãñ ç·¤ ?z?/2 ãñ, Ìæð
1
z
2
1 ·¤æ ‹ØêÙÌ× ×æÙ Ñ
(1)
5
2
âð çÙÚ´UÌÚU ÕǸæ ãñÐ
(2)
3
2
âð çÙÚ´UÌÚU ÕǸæ ãñ ÂÚU‹Ìé
5
2
âð ·¤× ãñÐ
(3)
5
2
·ð¤ ÕÚUæÕÚU ãñÐ
(4) ¥´ÌÚUæÜ (1, 2) ×ð´ çSÍÌ ãñÐ
63. ØçÎ a e R ÌÍæ â×è·¤ÚU‡æ
23(x2[x])212 (x2[x])1a250
(Áãæ¡ [x] ©â ÕǸð âð ÕǸð Âê‡ææZ·¤ ·¤æð ÎàææüÌæ ãñ Áæð
[ x ãñ) ·¤æ ·¤æð§ü Âê‡ææZ·¤èØ ãÜ Ùãè´ ãñ, Ìæð a ·ð¤ âÖè
â´Öß ×æÙ çÁâ ¥´ÌÚUæÜ ×ð´ çSÍÌ ãñ´, ßã ãñ Ñ
(1) (22, 21)
(2) (2:, 22) È (2, :)
(3) (21, 0) È (0, 1)
(4) (1, 2)](https://image.slidesharecdn.com/06-150313233700-conversion-gate01/85/06-04-2014-e-28-320.jpg)


![SPACE FOR ROUGH WORK / ÚUȤ ·¤æØü ·ð¤ çÜ° Á»ãE/Page 31
70.
2
20
sin ( cos )
x
x
lim
x
p
“
is equal to :
(1) 2p
(2) p
(3)
2
p
(4) 1
71. If g is the inverse of a function f and
f 9 (x)5 5
1
1 x1
, then g9 (x) is equal to :
(1)
{ }5
1
1 ( )g x1
(2) 11{g(x)}5
(3) 11x5
(4) 5x4
72. If f and g are differentiable functions in
[0, 1] satisfying f (0)525g(1), g(0)50 and
f (1)56, then for some ce]0, 1[ :
(1) f 9(c)5g9(c)
(2) f 9(c)52g9(c)
(3) 2f 9(c)5g9(c)
(4) 2f 9(c)53g9(c)
70.
2
20
sin ( cos )
x
x
lim
x
p
“
·¤æ ×æÙ ãñ Ñ
(1) 2p
(2) p
(3)
2
p
(4) 1
71. ØçÎ g ȤÜÙ f ·¤æ ÃØ鈷ý¤× ãñ ÌÍæ f9 (x)5 5
1
1 x1
ãñ, Ìæð g9 (x) ÕÚUæÕÚU ãñ Ñ
(1)
{ }5
1
1 ( )g x1
(2) 11{g(x)}5
(3) 11x5
(4) 5x4
72. ØçÎ f ÌÍæ g, [0, 1] ×𴠥߷¤ÜÙèØ È¤ÜÙ ãñ´ Áæð
f (0)525g(1), g(0)50 ¥æñÚU f (1)56 ·¤æð â´ÌécÅU
·¤ÚUÌð ãñ´, Ìæð ç·¤âè ce]0, 1[ ·ð¤ çÜ° Ñ
(1) f 9(c)5g9(c)
(2) f 9(c)52g9(c)
(3) 2f 9(c)5g9(c)
(4) 2f 9(c)53g9(c)](https://image.slidesharecdn.com/06-150313233700-conversion-gate01/85/06-04-2014-e-31-320.jpg)