Decoding the Latest Evidence and Practical Recommendations on Biomarker Testing for New Therapeutic Options Targeting HER2, HER3, and TROP2 in Solid Tumors
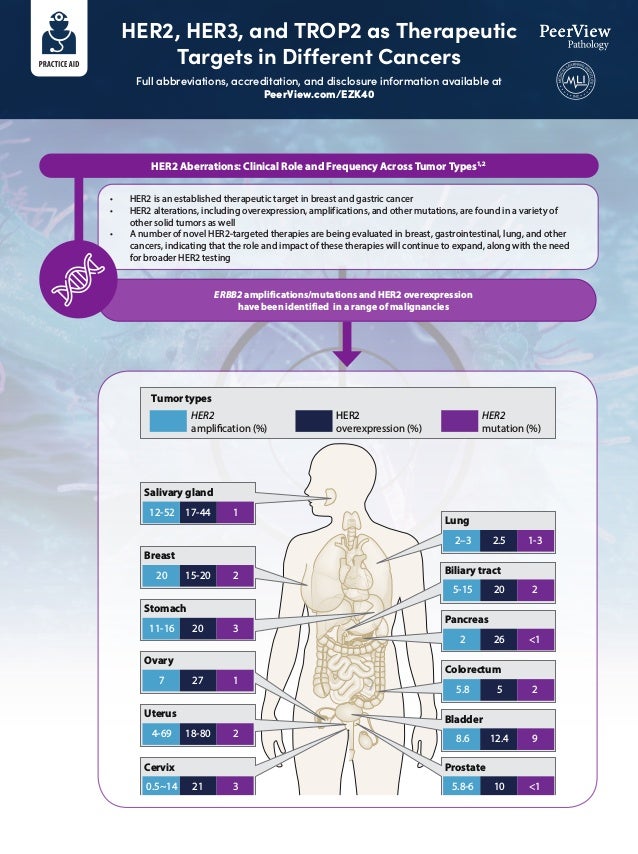
- 1. HER2, HER3, and TROP2 as Therapeutic Targets in Different Cancers Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 HER2 Aberrations: Clinical Role and Frequency Across Tumor Types1,2 • HER2 is an established therapeutic target in breast and gastric cancer • HER2 alterations, including overexpression, amplifications, and other mutations, are found in a variety of other solid tumors as well • A number of novel HER2-targeted therapies are being evaluated in breast, gastrointestinal, lung, and other cancers, indicating that the role and impact of these therapies will continue to expand, along with the need for broader HER2 testing ERBB2 amplifications/mutations and HER2 overexpression have been identified in a range of malignancies Colorectum 5.8 5 2 Pancreas 2 26 1 Ovary 7 27 1 Prostate 5.8-6 10 1 Tumor types HER2 amplification (%) HER2 overexpression (%) HER2 mutation (%) Breast 20 15-20 2 Salivary gland 12-52 17-44 1 Stomach 11-16 20 3 Bladder 8.6 12.4 9 Cervix 0.5~14 21 3 Uterus 4-69 18-80 2 Lung 2–3 2.5 1-3 Biliary tract 5-15 20 2
- 2. HER2, HER3, and TROP2 as Therapeutic Targets in Different Cancers Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 1. Oh DY, Bang YJ. Nat Rev Clin Oncol. 2020;17:33-48. 2. Hechtman JF, Ross DS. Cancer Cytopathol. 2019;127:428-431. 3. Mishra R et al. Oncol Rev. 2018;12:355. 4. Guerra E et al. Oncogene. 2013;32:1594-1600. 5. Zeng P et al. Sci Rep. 2016;6:33658. Mechanism of Action of Agents Targeting HER21 HER3 and TROP2 as Emerging Therapeutic Targets a. Single-epitope monoclonal antibodies bind HER2 at a single extracellular domain, inhibiting downstream signaling, engaging antibody-dependent cytotoxicity, or inhibiting receptor dimerization b. ADCs also have antitumor effects through these pathways, but they additionally exhibit cytotoxicity by releasing a cytotoxic agent close to HER2-positive tumor cells c. Bispecific antibodies target more than one extracellular region of HER2 d. Small-molecule inhibitors bind the intracellular tyrosine-kinase domain HER33 • Crucial heterodimeric partner for other EGFR family members • Potential to regulate EGFR/HER2-mediated resistance • Enhanced expression associated with several cancers, including lung, breast, ovarian, prostate, gastric, bladder, melanoma, colorectal, and squamous cell carcinoma • Implicated in contributing to treatment failure through activation of PI3K/AKT, MAPK/ERK, and JAK/STAT pathways • HER3-targeting investigational therapies: mono and bispecific antibodies targeting HER3 at multiple subdomains; miscellaneous HER3-targeting therapies, including antisense oligonucleotides, HER3-specific peptide vaccines, ligand traps, molecules targeting HER3 pseudokinase activity, pan-HER approaches, HER3 ADCs, and HER3 nanobiologic therapeutic approaches; select examples: patritumab deruxtecan/U3-1402 (HER3-targeting ADC) and MCLA-128 (HER2–HER3 bispecific antibody) TROP24,5 • Transmembrane glycoprotein overexpressed in many different tumors, including lung, breast, pancreatic, cervical, ovarian, colorectal, and gastric cancers • Membrane bound with an extracellular domain • Effectively internalized with binding antibody • High expression correlates with poor prognosis • Rational therapeutic target; TROP2-targeting therapies: sacituzumab govitecan/IMMU-132, datopotamab deruxtecan/DS-1062 Pertuzumab a. Single-Epitope mAbs b. ADCs d. Small-Molecule Inhibitors c. Bispecific Antibodies Dimerization domain Trastuzumab Margetuximab Inhibition of receptor dimerization Targeted delivery of highly cytotoxic agents Direct inhibition of the downstream tyrosine-kinase domain Promotion of receptor internalization and/or degradation Engagement of ADCC Tyrosine-kinase domain Cell membrane Trastuzumab emtansine Trastuzumab deruxtecan Dual targeting of the trastuzumab and pertuzumab binding sites ZW25 Lapatinib Neratinib Tucatinib I I I III IV I I I III IV Inhibition of PI3-kinase signalling promoting cell-cycle arrest I I I III IV
- 3. Treatment Recommendations for HER2+ Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 a Alternative taxanes (ie, docetaxel, paclitaxel, albumin-bound paclitaxel) may be substituted for select patients due to medical necessity (ie, hypersensitivity reaction). If substituted for weekly paclitaxel or docetaxel, then the weekly dose of albumin-bound paclitaxel should not exceed 125 mg/m2 . b Paclitaxel + trastuzumab may be considered for patients with low-risk T1, N0, M0, HER2+ disease, particularly those not eligible for other standard adjuvant regimens due to comorbidities. c Trastuzumab given in combination with an anthracycline is associated with significant cardiac toxicity. Concurrent use of trastuzumab and pertuzumab with an anthracycline should be avoided. d Consider extended adjuvant neratinib following adjuvant trastuzumab-containing therapy for patients with HR+/HER2+ with a perceived high risk of recurrence. The benefit or toxicities associated with extended neratinib in patients who have received pertuzumab or ado-trastuzumab emtansine is unknown. e It is acceptable to change the administration sequence to taxane (with or without HER2-targeted therapy) followed by AC. 1. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Preferred Regimens • Paclitaxel + trastuzumabb • TCH (docetaxel/carboplatin/trastuzumab) • TCHP (docetaxel/carboplatin/trastuzumab/pertuzumab) • If no residual disease after preoperative therapy or no preoperative therapy: complete up to 1 year of HER2-targeted therapy with trastuzumabc (category 1) ± pertuzumab • If residual disease after preoperative therapy: trastuzumab emtansine (category 1) alone; if trastuzumab emtansine discontinued for toxicity, then trastuzumab (category 1) ± pertuzumab to complete 1 year of therapyc,d Useful in Certain Circumstances Other Recommended Regimens • Docetaxel + cyclophosphamide + trastuzumab • AC followed by Te + trastuzumabc (doxorubicin/cyclophosphamide followed by paclitaxel + trastuzumab; various schedules) • AC followed by Te + trastuzumab + pertuzumabc (doxorubicin/cyclophosphamide followed by paclitaxel + trastuzumab + pertuzumab; various schedules) • Neratinibd (adjuvant setting only) • Paclitaxel + trastuzumab + pertuzumabc • Trastuzumab emtansine (adjuvant setting only) • AC followed by docetaxele + trastuzumabc (doxorubicin/cyclophosphamide followed by docetaxel + trastuzumab) • AC followed by docetaxele + trastuzumab + pertuzumabc (doxorubicin/cyclophosphamide followed by docetaxel + trastuzumab + pertuzumab) Preoperative/Adjuvant Therapy: Updated Recommendations Based on NCCN Guidelines Version 1.20221,a Updates in Version 1.2022 of the Guidelines From Version 8.2021 Useful in certain circumstances, options added: • Neratinib (adjuvant setting only) • Paclitaxel + trastuzumab + pertuzumab • Trastuzumab emtansine (adjuvant setting only) !
- 4. Treatment Recommendations for HER2+ Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 a Maintenance trastuzumab/pertuzumab after response (with concurrent endocrine therapy if ER+ and HER2+ metastatic breast cancer). b Regimens may also be used as an option for third line and beyond; the optimal sequence for third-line therapy and beyond is not known. c An FDA-approved biosimilar is an appropriate substitute for trastuzumab. d Trastuzumab deruxtecan may be considered in the first-line setting as an option for select patients (ie, those with rapid progression within 6 months of neoadjuvant or adjuvant therapy [12 months for pertuzumab-containing regimens]). e Trastuzumab deruxtecan is contraindicated for patients with pneumonitis or ILD. f Tucatinib + trastuzumab + capecitabine is preferred in patients with both systemic and CNS progression in the third-line setting and beyond and it may be given in the second-line setting. g Multiple lines of concurrent chemotherapy with anti-HER2 therapy (trastuzumab or a TKI) offer clinical benefit for recurrent unresectable HER2+ metastatic breast cancer and have been studied in phase 2 or 3 trials. Clinical experience suggests frequent clinical benefit for such treatment. However, there are no meaningful data for use of any of these regimens among patients previously treated with pertuzumab-based chemotherapy, trastuzumab emtansine, trastuzumab deruxtecan, or trastuzumab/capecitabine/tucatinib regimens. Thus, the optimal sequence or true benefit of therapy is not known. h Trastuzumab given in combination with an AC is associated with significant cardiac toxicity. Concurrent use of trastuzumab and pertuzumab with an AC should be avoided. i Trastuzumab may be safely combined with all non−AC-containing preferred and other single agents listed on the NCCN guidelines for systemic therapies for recurrent or metastatic breast cancer (BINV-Q [1 of 8]). 1. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Updates in Version 1.2022 of the Guidelines From Version 8.2021 • Second-line option added: Trastuzumab deruxtecan; this is a category 1 preferred regimen • Second-line option modified: Trastuzumab emtansine has been changed from a category 1 preferred regimen to a category 2A other recommended regimen • Heading modified: Third line and beyond (optimal sequence is not known) Recurrent Unresectable (Local or Regional) or Stage IV (M1) Disease: Updated Recommendations Based on NCCN Guidelines Version 1.20221 Setting Regimen NCCN Category of Preference Evidence Level First linea Pertuzumab + trastuzumab + docetaxelc Preferred regimen 1 Pertuzumab + trastuzumab + paclitaxelc Preferred regimen 2A Second lineb Trastuzumab deruxtecan (T-DXd)b,d,e Preferred regimen 1 Trastuzumab emtansine (T-DM1)b Other recommended regimen 2A Third line and beyond (optimal sequence unknown) Tucatinib + capecitabine + trastuzumabc,f Other recommended regimen 1 Trastuzumab + docetaxel or vinorelbinec,g Other recommended regimen 2A Trastuzumab + paclitaxel ± carboplatinc,g Other recommended regimen 2A Capecitabine + trastuzumab or lapatinibc,g Other recommended regimen 2A Trastuzumab + lapatinibc,g (without cytotoxic therapy) Other recommended regimen 2A Trastuzumab + other agentsc,g,h,i Other recommended regimen Neratinib + capecitabineg Other recommended regimen 2A Margetuximab + chemotherapyg (capecitabine, eribulin, gemicitabine, or vinorelbine) Other recommended regimen 2A 2A • Footnote b modified: Regimens may also be used as an option for third line and beyond or fourth-line option; the optimal sequence for third-line therapy and beyond is not known • Footnote d added: Trastuzumab deruxtecan may be considered in the first-line setting as an option for select patients (ie, those with rapid progression within 6 months of neoadjuvant or adjuvant therapy [12 months for pertuzumab-containing regimens]) • Footnote f modified: Tucatinib + trastuzumab + capecitabine is preferred in patients with both systemic and CNS progression in the third-line setting and beyond on trastuzumab emtansine. However, tucatinib + trastuzumab + capecitabine and it may be given in the second-line setting • Footnote removed: Trastuzumab deruxtecan is preferred in patients with visceral metastases if progression on trastuzumab emtansine !
- 5. Testing for HER2 and HER2-Low Expression in Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 Must order reflex test (same specimen using ISH) or order a new test (new specimen if available, using IHC or ISH) IHC 3+ positive IHC 1+ negative IHC 0 negative No staining is observed or Membrane staining that is faint/barely perceptible and in ≤10% of tumor cells IHC 2+ equivocal Batch controls and on-slide controls show appropriate staining Incomplete membrane staining that is faint/barely perceptible and in 10% of tumor cells Weak-to-moderate complete membrane staining observed in 10% of tumor cells Circumferential membrane staining that is complete, intense, and in 10% of tumor cells HER2 testing (invasive component) by validated IHC assay • HER2 IHC 2+ (equivocal) cases with “weak-to-moderate complete membrane staining” observed in 10% of tumor cells • If the initial HER2 test result in a core needle biopsy is negative, may repeat test in excision specimen based on clinical criteria • If HER2/CEP17 ratio of ≥2.0, but the average HER2 signals per cell is 4.0, additional workup is needed • If average of ≥6.0 HER2 signals per cell with an HER2/CEP17 ratio 2.0 (formerly diagnosed as ISH positive for HER2), additional workup is needed • If average HER2 signals per tumor cell ≥4.0 and 6.0 and the HER2/CEP17 ratio is 2.0 (formerly diagnosed as ISH equivocal), additional workup is needed Updates in the 2018 ASCP/CAP Guidelines1 Recommendations by the ASCO/CAP HER2 Testing Expert Panel are aimed at improving the analytic validity of HER2 testing and the clinical utility of HER2 as a predictive biomarker for potential responsiveness to therapies targeting the HER2 protein. HER2 gene amplification assessed by in situ hybridization (ISH) or protein overexpression assessed by IHC remains the primary predictor of responsiveness to HER2-targeted therapies in breast cancer. Breast Cancer: 2018 ASCO/CAP Guidelines for Evaluation of HER2 Protein Expression by IHC1
- 6. Testing for HER2 and HER2-Low Expression in Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 ISH positive ISH negative Evaluation of HER2 Gene Amplification by ISH Assay Using Dual-Probe ISH¹ Additional workup required Additional workup required Additional workup required HER2 testing (invasive component) by validated dual-probe ISH assay Evaluation of HER2 Gene Amplification by ISH Assay Using Single-Probe ISH¹ HER2/CEP17 ratio ≥2.0 HER2/CEP17 ratio 2.0 Group 1 Average HER2 copy number ≥4.0 signals/cell Group 2 Average HER2 copy number 4.0 signals/cell Group 3 Average HER2 copy number ≥6.0 signals/cell Group 4 Average HER2 copy number ≥4.0 and 6.0 signals/cell Group 5 Average HER2 copy number 4.0 signals/cell Batch controls and on-slide controls show appropriate hybridization ISH positive ISH negative Average HER2 copy number ≥6.0 signals/cell Average HER2 copy number 4.0 signals/cell Concurrent IHC 3+ and/or Concurrent dual-probe ISH group 1 Concurrent IHC 0, 1+ and/or Concurrent dual-probe ISH group 5 Batch controls and on-slide controls show appropriate hybridization Average HER2 copy number ≥4.0 and 6.0 signals/cell Perform dual-probe ISH for final result Concurrent IHC 2+ HER2 testing (invasive component) by validated single-probe ISH assay
- 7. Testing for HER2 and HER2-Low Expression in Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 Summary of the Major Changes in the ASCO/CAP HER2 Testing Guidelines Between 2007 and 2018² HER2 testing must be performed on all newly diagnosed and recurrent breast cancers Re-emphasized the importance of testing metastatic breast cancers At least one HER2 test should be performed on primary and metastatic breast cancers Specimen type Same as 2013 Homogeneous, dark circumferential membrane staining in 10% of invasive tumor cells Homogeneous, dark circumferential membrane staining in 30% of invasive tumor cells IHC positive HER2 (3+) Same as 2013 • 0: No staining or incomplete membranous staining that is faint/barely perceptible and within ≤10% of invasive tumor cells • 1+: Incomplete membranous staining that is faint/barely perceptible and within 10% of invasive tumor cells • 0: No staining • 1+: Weak incomplete membrane staining in any proportion of tumor cells or weak, complete membrane staining in 10% IHC negative HER2 (0 or 1+) Same as 2013 • Single probe: HER2 copy number ≥4.0 and 6 signals/cell • Dual probe: HER2/CEP17 ratio of 2.0 with an average HER2 copy number ≥4.0 and 6 signals/cell HER2/CEP17 ratio of 1.8-2.2 or average HER2 gene copy number 4-6 signals/nucleus for test without an internal control probe ISH equivocal Need additional work concomitant with IHC result to render a diagnosis whether HER2 is positive or negative, with an explanatory comment to be added Must repeat if: • Tumor on excision is grade 3 • Invasive tumor in the NCB is small • Resection specimen contains high-grade carcinoma that is morphologically distinct from the prior core • Core biopsy result is equivocal for HER2 after testing by both ISH and IHC • There is doubt about the specimen handling of the core biopsy (long ischemic time, short time in fixative, different fixative) or the test is suspected by the pathologist to be negative on the basis of testing error No recommendations NCB negative Same as 2013 but change the word “must” to “may” 2013 2007 Item 2018 Circumferential membrane staining that is incomplete and/or weak/moderate and with 10% of invasive tumor cells Complete and circumferential membranous staining that is intense and within ≤10% of invasive tumor cells Non-uniform or weak intensity, circumferential, complete membranous staining in at least 10% of invasive tumor cells Complete and intense membranous staining of 30% or less of invasive tumor cells Complete weak/moderate membrane staining in 10% of invasive tumor cells Complete and circumferential membranous staining that is intense and within ≤10% of invasive tumor cells • Single probe: HER2 copy number 4.0 signals/cell • Dual probe: HER2/CEP17 ratio of 2.0 with an average HER2 copy number 4.0 signals/cell HER2/CEP17 ratio of 1.8 or average HER2 gene copy number 4 signals/nucleus for test without an internal control probe Same as 2013 IHC equivocal HER2 (2+) No specification on the type of the probe (whether single or dual) for test without an internal control probe • HER2 to CEP17 ratio of 2.2 or average HER2 gene copy number 6 signals/nucleus ISH positive Same as 2013 for group 1 Groups 2 and 3: Need additional work concomitant with IHC result to render a diagnosis whether HER2 is positive or negative, with an explanatory comment to be added Specified criteria for single and dual probe: • Group 1: Single-probe average HER2 copy number ≥6.0 signals/cell or dual-probe HER2/CEP17 ratio of ≥2.0 with an average HER2 copy number ≥4.0 signals/cell • Group 2: Dual-probe HER2/CEP17 ratio of ≥2.0 with an average HER2 copy number 4.0 signals/cell • Group 3: Dual-probe HER2/CEP17 ratio of 2.0 with an average HER2 copy number ≥6.0 signals/cell ISH negative 6-72 h 6-48 h Same as 2013 Duration of tissue fixation
- 8. Testing for HER2 and HER2-Low Expression in Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 Going Beyond the Dichotomous HER2+/- Status? Proposed Algorithm for Defining HER2-Low Breast Cancer4 HER2 positive HER2 low HER2 negative Reflex ISH test positive Reflex ISH test negative Circumferential membrane staining that is complete, intense, and in 10% of tumor cells IHC 3+ Weak-to-moderate complete membrane staining in 10% of tumor cells IHC 2+ Incomplete membrane staining that is faint/barely perceptible and in 10% of tumor cells IHC 1+ No staining is observed: HER2 null or Membrane staining that is incomplete and is faint/barely perceptible and in10% of tumor cells IHC 0+ HER2 testing by validated IHC assay HER2 positive HER2 negative Dichotomous HER2 positive HER2 negative HER2 low New paradigm? Challenge: Distinguishing IHC score 0 from score 1+ HER-low BC: ≈55% • IHC 1+, IHC 2+ with a negative ISH test HER2-positive BC: ≈15% HER2-negative BC: ≈30% International guidelines currently recommend a binary model (HER2 positive vs negative) to guide clinicians in treatment decisions. However, a great proportion of patients (≈40%-55%) classified as HER2 negative are, in fact, HER2 low; a population with a high unmet medical need. Despite past drawbacks with older drugs, a new generation of anti-HER2 agents has recently shown encouraging signs of clinical activity and safety in HER2-low disease.3
- 9. Testing for HER2 and HER2-Low Expression in Breast Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 1. Wolff AC et al. Arch Pathol Lab Med. 2018;142:1364-1382. 2. Zhang H et al. Curr Oncol Rep. 2020;22:51. 3. Eiger D et al. Cancers (Basel). 2021;13:1015. 4. Tarantino P et al. J Clin Oncol. 2020;38:1951-1962. Novel Agents and Mechanisms Enabling the Targeting of HER2-Low Breast Cancer4 Th1 Vaccines Bispecific Antibodies Antibody–Drug Conjugates Monoclonal Antibodies Trastuzumab Margetuximab Pertuzumab RAF/MEK/MAPK Proliferation PI3K/AKT/mTOR Survival Trastuzumab Pertuzumab Margetuximab TrasGEX Trastuzumab deruxtecan Trastuzumab duocarmazine PF-06804103 A166 RC48-ADC ARX788 Antitumoral agent Linker Antibody Proliferation Survival MCLA-128 ZW25 Ertumaxomab MM-111 GBR 1302 Nelipepimut-S GP2 AE37 CTL MHC I TNFα IFNγ HER2- polarized DC Novel anti-HER2 drugs enable the targeting of low HER2-expressing cancers through different mechanisms: A. Monoclonal antibodies engineered to enhance antibody-dependent cellular cytotoxicity, or to more effectively inhibit HER2 heterodimerization, have shown preclinical evidence of activity in HER2-low breast cancer. B. Novel antibody–drug conjugates enable exploitation of low HER2 expression to direct cytotoxic molecules to tumor cells, showing promising activity in early clinical trials. C. Bispecific antibodies allow forced connections between cancer and immune cells and/or suppress multiple signaling pathways, with potential activity in HER2-low breast cancer cells. D. Cancer vaccines enhance antitumor immune response against HER2 and are currently being tested in the adjuvant setting to reduce relapses in HER2-low breast cancer. A B C D
- 10. HER2 Testing in Gastroesophageal Cancer Guidance From CAP/ASCP/ASCO1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 GEA and potential candidate for HER2-targeted therapy HER2-targeted therapy should not be initiated until HER2 positivity is confirmed Biopsy or resection specimen from primary or metastatic sites should be used Alternative: FNA specimens (cell blocks) may be used Request HER2 test Equivocal or negative result Inadequate specimen tested No positive results Positive results Initiate HER2-targeted therapy; no further HER2 testing is required Retest additional available tissue; if there is no available tissue, additional tumor tissue may be obtained for retesting NGS2 enables assessment of ERBB2 copy number and mutations simultaneously, along with other molecular events such as TMB and MSI status; can be performed on both surgical specimens or cell blocks and has recently been shown to work with cytologic supernatant
- 11. HER2 Testing in Gastroesophageal Cancer Guidance From CAP/ASCP/ASCO1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 Perform HER2 test using IHC IHC 2+ Equivocal IHC 1+ Negative IHC 3+ Positive Perform ISH Testing IHC 0 Negative Surgical specimen Strong, complete basolateral or lateral membranous reactivity in ≥10% of tumor cells Biopsy specimen Tumor cell cluster with strong, complete basolateral or lateral membranous activity irrespective of percentage of tumor cells stained Surgical specimen Weak to moderate, complete basolateral or lateral membranous reactivity in ≥10% of tumor cells Biopsy specimen Tumor cell cluster with weak/ moderate, complete basolateral or lateral membranous activity irrespective of percentage of tumor cells stained Surgical specimen Faint/barely perceptible membranous reactivity in ≥10% of tumor cells; cells reactive only in part of their membrane Biopsy specimen Tumor cell cluster with faint/barely membranous activity irrespective of percentage of tumor cells stained Surgical specimen No reactivity or membranous reactivity in 10% of tumor cells Biopsy specimen No reactivity in any tumor cells No further ISH testing is required No further ISH testing is required Tissue sample from patient diagnosed with GEA
- 12. HER2 Testing in Gastroesophageal Cancer Guidance From CAP/ASCP/ASCO1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 1. Bartley AN et al. Am J Clin Pathol. 2016;146:647-669. 2. Hechtman JF, Ross DS. Cancer Cytopathol. 2019;127:428-431. Summary of HER2 Testing Recommendations Strength of Recommendation Strong recommendation • In patients with GEA who are potential candidates for HER2-targeted therapy, the treating clinician should request HER2 testing on tumor tissue Strong recommendation • Laboratories must incorporate GEA HER2 testing methods into their overall laboratory quality improvement program, establishing appropriate quality improvement monitors as needed to ensure consistent performance in all steps of the testing and reporting process; in particular, laboratories performing GEA HER2 testing must participate in a formal proficiency testing program, if available, or an alternative proficiency assurance activity • Laboratories should report HER2 testing results in GEA specimens in accordance with the CAP “Template for Reporting Results of HER2 (ERBB2) Biomarker Testing of Specimens From Patients With Adenocarcinoma of the Stomach or Esophagogastric Junction” Strong recommendation Strong recommendation • Pathologists should identify areas of invasive adenocarcinoma and also mark areas with the strongest intensity of HER2 expression by IHC in GEA specimens for subsequent ISH scoring when required Recommendations No recommendation • There is insufficient evidence to recommend for or against genomic testing in patients with GEA at this time Strong recommendation • When GEA HER2 status is being evaluated, laboratories/pathologists should perform/order IHC testing first, followed by ISH when IHC result is 2+ (equivocal); positive (3+) or negative (0 or 1+) HER2 IHC results do not require further ISH testing • Pathologists should select the tissue block with the areas of lowest grade tumor morphology in biopsy and resection specimens; more than 1 tissue block may be selected if different morphologic patterns are present Recommendation • Pathologists should use the Ruschoff/Hofmann method in scoring HER2 IHC and ISH results for GEA Strong recommendation Strong recommendation • Laboratories/pathologists must specify the antibodies and probes used for the test and ensure that assays are appropriately validated for HER2 IHC and ISH on GEA specimens Recommendation • Treating clinicians should offer combination chemotherapy and HER2-targeted therapy as the initial treatment for appropriate patients with HER2-positive tumors who have metastatic or recurrent GEA Recommendation • Treating clinicians or a pathologist should request HER2 testing on tumor tissue in the biopsy or resection specimens (primary or metastasis), preferably before the initiation of trastuzumab therapy if such specimens are available and adequate; HER2 testing on FNA specimens (cell blocks) is an acceptable alternative
- 13. HER2, HER3, and TROP2 as Therapeutic Targets in Lung Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 • HER2 is overexpressed, amplified, or mutated in a significant fraction of lung adenocarcinomas, but prognostic significance is not yet fully defined – Frequency of overexpression (IHC 2+ and 3+): 15%-30% – Frequency of overexpression (IHC 3+): 2%-6% – Frequency of amplification: 2%-6% – Frequency of mutations: 1%-5% • HER2 dysregulations encompass heterogeneous and distinct alterations which must be taken into consideration – There is a lack of correlation between overexpression, amplification, and mutations – Cohorts must be defined according to the specific HER2 alteration present • The appropriate testing methodology for different HER2 alterations should be used – Amplification: in situ hybridization (ISH) – Expression: immunohistochemistry (IHC) – Mutation: sequencing (NGS or other) • Although they have become outdated in the context of the rapid advances in targeted therapy for lung cancer and related biomarker testing demands, the latest published lung cancer molecular testing guidelines state that HER2 molecular testing is not indicated as a routine stand-alone assay to guide targeted therapy selection outside the context of a clinical trial. However, it is appropriate to include HER2 mutation analysis as part of a larger testing panel performed either initially or when routine testing results for other alterations with approved targeted therapies are negative. • There is a rationale for investigating HER2-targeted therapies in NSCLC • Antibody–drug conjugates (ADCs) represent a promising therapeutic approach; agents under investigation include: – Trastuzumab deruxtecan (T-DXd)—in various settings of HER2-expressing and HER2-mutated NSCLC – Ado-trastuzumab emtansine (T-DM1) • HER3 is highly expressed in NSCLC • HER3 overexpression is associated with metastatic progression and poor outcomes in patients with NSCLC • There are currently no established biomarker testing recommendations for HER3 in NSCLC • HER3 alterations are not known to be a mechanism of resistance to EGFR TKIs in EGFR-mutated NSCLC • Targeting HER3 may address multiple EGFR-targeted therapy resistance mechanisms • There is a rationale for investigating HER3-targeted therapies in NSCLC • ADCs represent a promising therapeutic approach: eg, anti-HER3 ADC patritumab deruxtecan (HER3-DXd) is under investigation in various settings of EGFR-mutated advanced NSCLC HER1 HER2 HER3 HER4 EGFR family Cytoplasm Nucleus Chr17 HER2 • Proliferation • Motility • Invasiveness • Survival • Angiogenesis P P PI3K Akt SOS RAS RAF MEK MAPK P P Ligands Role of HER2 in NSCLC1-7 Role of HER3 in NSCLC8
- 14. HER2, HER3, and TROP2 as Therapeutic Targets in Lung Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/EZK40 Role of TROP2 in NSCLC9-12 • TROP2 is highly expressed in NSCLC and has been associated with poor prognosis • There are currently no established biomarker testing recommendations for TROP2 in NSCLC • There is a rationale for investigating TROP2-targeted therapies in NSCLC • ADCs represent a promising therapeutic approach: eg, the anti-TROP2 ADC datopotamab deruxtecan (Dato-DXd) is under investigation in various settings of NSCLC with and without actionable genomic alterations 1. Li BT et al. J Thorac Oncol. 2016;11:414-419. 2. Li BT et al. International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer (WCLC 2017). Abstract OA14.05. 3. Li BT et al. J Clin Oncol. 2018;36:2532-2537. 4. Iqbal N et al. Mol Biol Int. 2014;2014:852748. 5. Yan M et al. Cancer Metastasis Rev. 2015;34:157-164. 6. Rolfo C et al. Cancer Discov. 2020;10:643-645. 7. Lindeman NI et al. Arch Pathol Lab Med. 2018;142:321-346. 8. Scharpenseel H et al. Sci Rep. 2019;9:7406. 9. Mito R et al. Pathol Int. 2020;70:287-294. 10. Inamura K et al. Oncotarget. 2017;8:28725-28735. 11. Jiang A et al. Oncol Lett. 2013;6:375-380. 12. Lenart S et al. Cancers (Basel). 2020;12:3328. Fibronectin Decreased adhesion Increased cell migration ErB3 activation NRG-1 is released when TROP2 is lost Recruitment to tight junctions NRG-1 TACE Clauidn 1/7 TROP2 α5 β1 Talin RACK 1 RACK 1 FAK P FAK Src Src P NRG-1 Endosome
