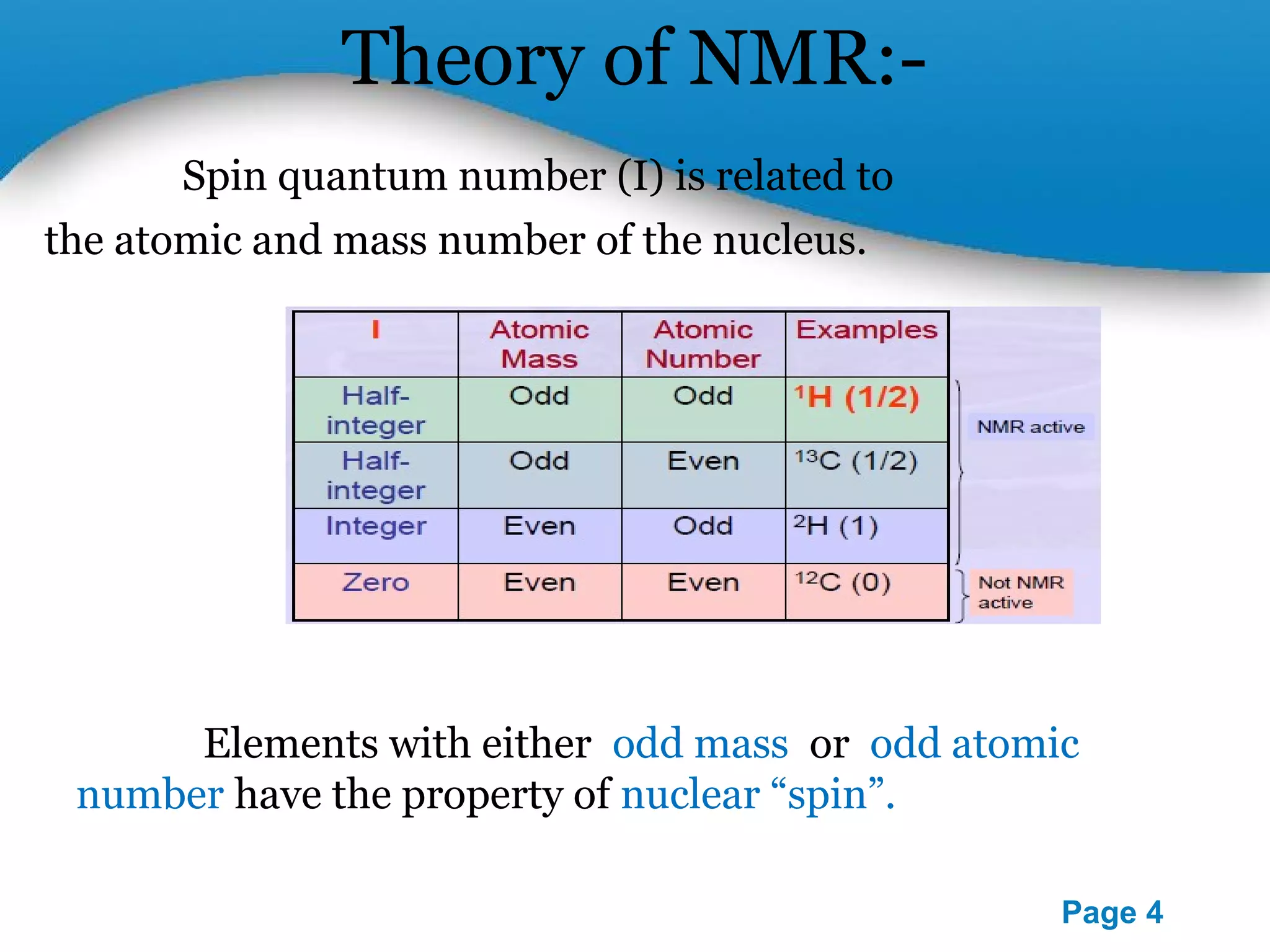
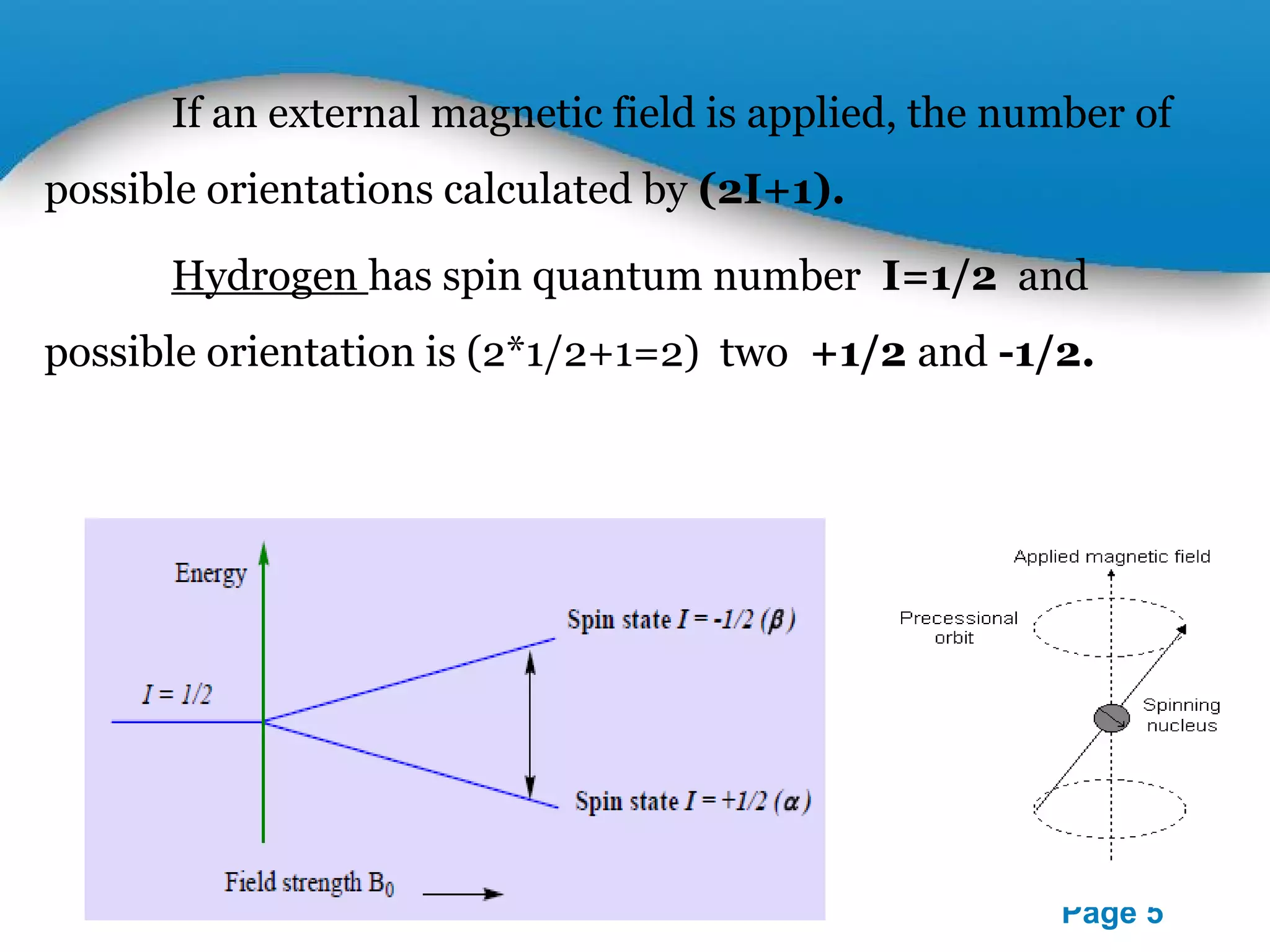
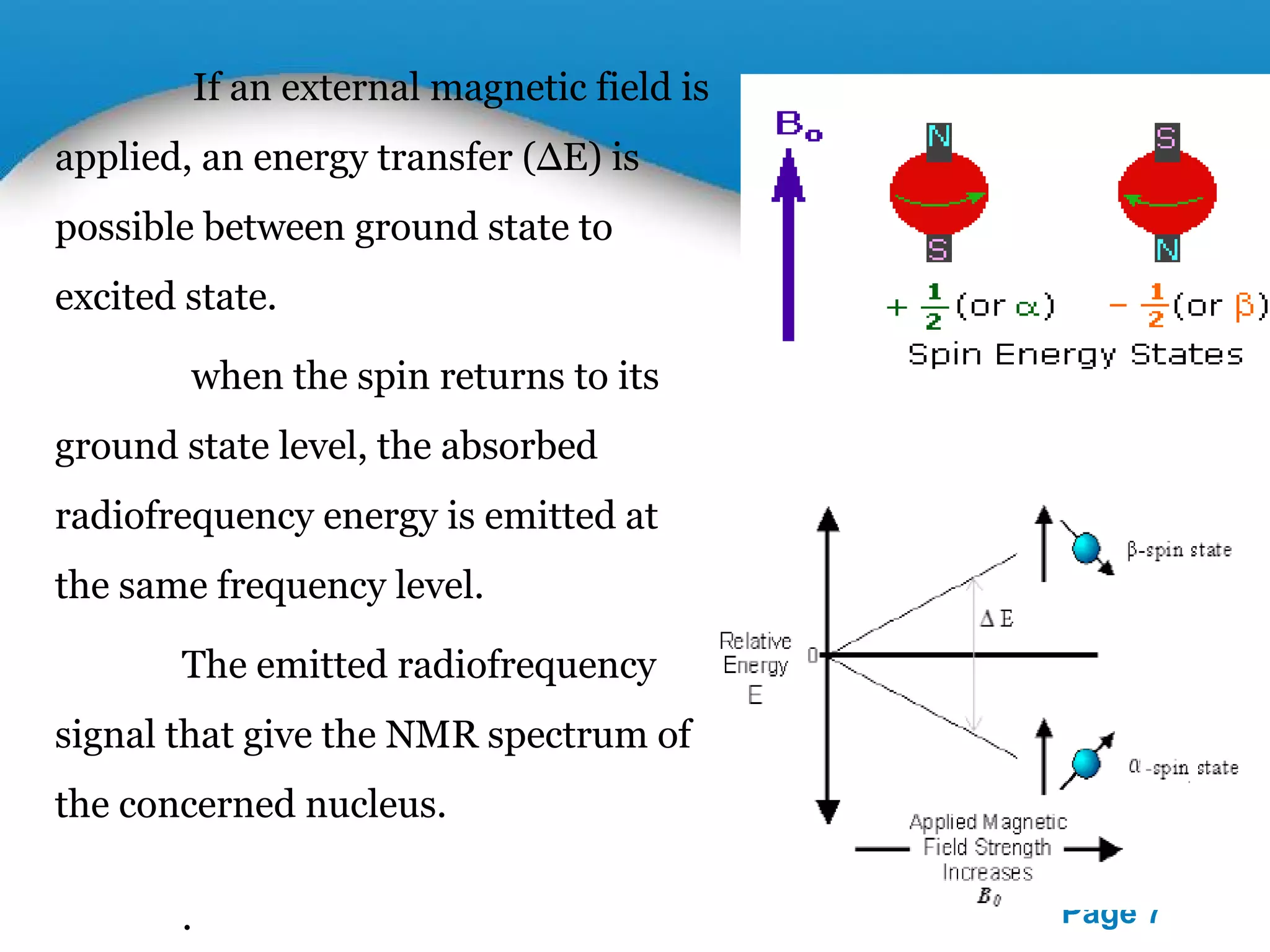
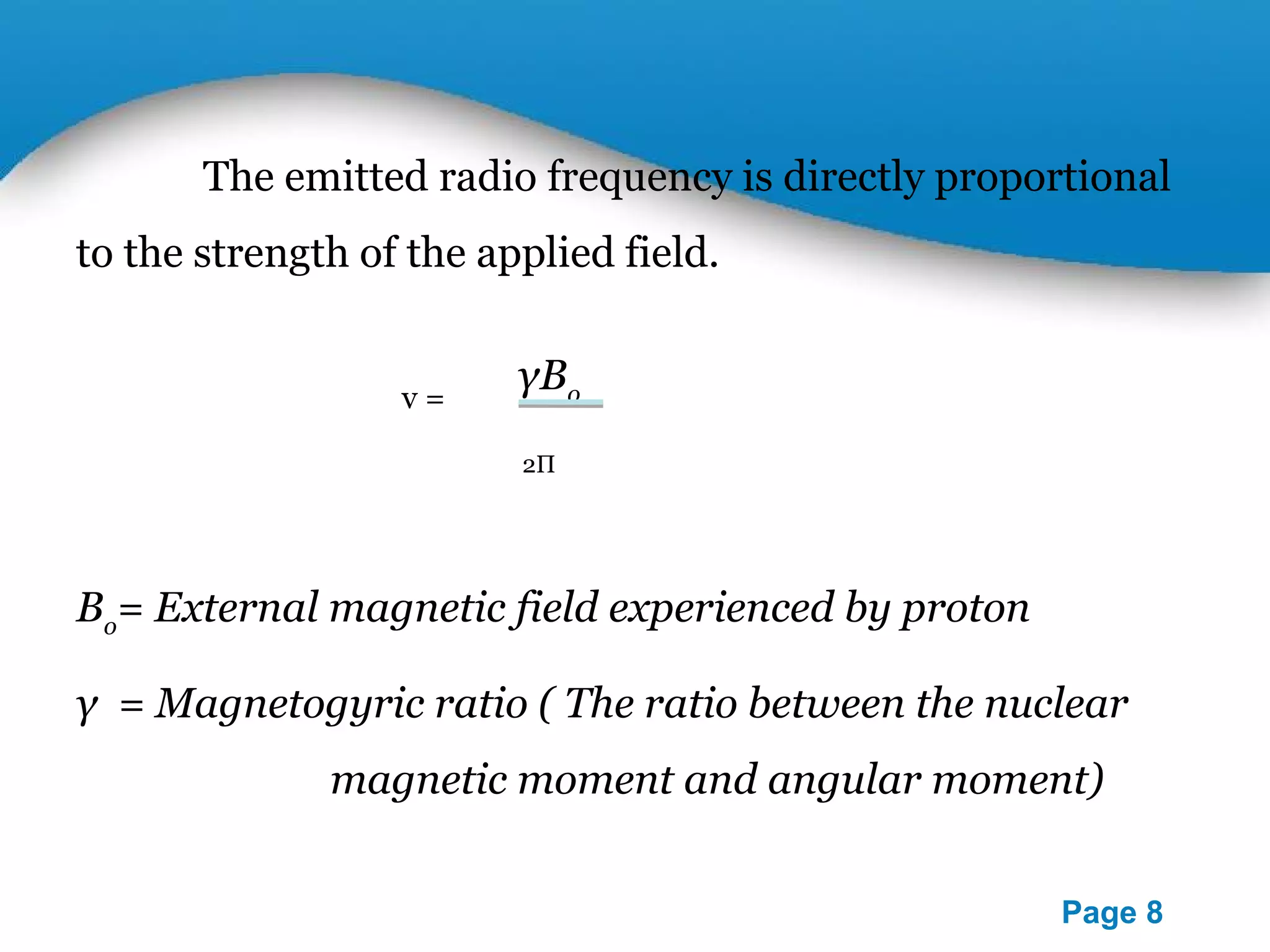
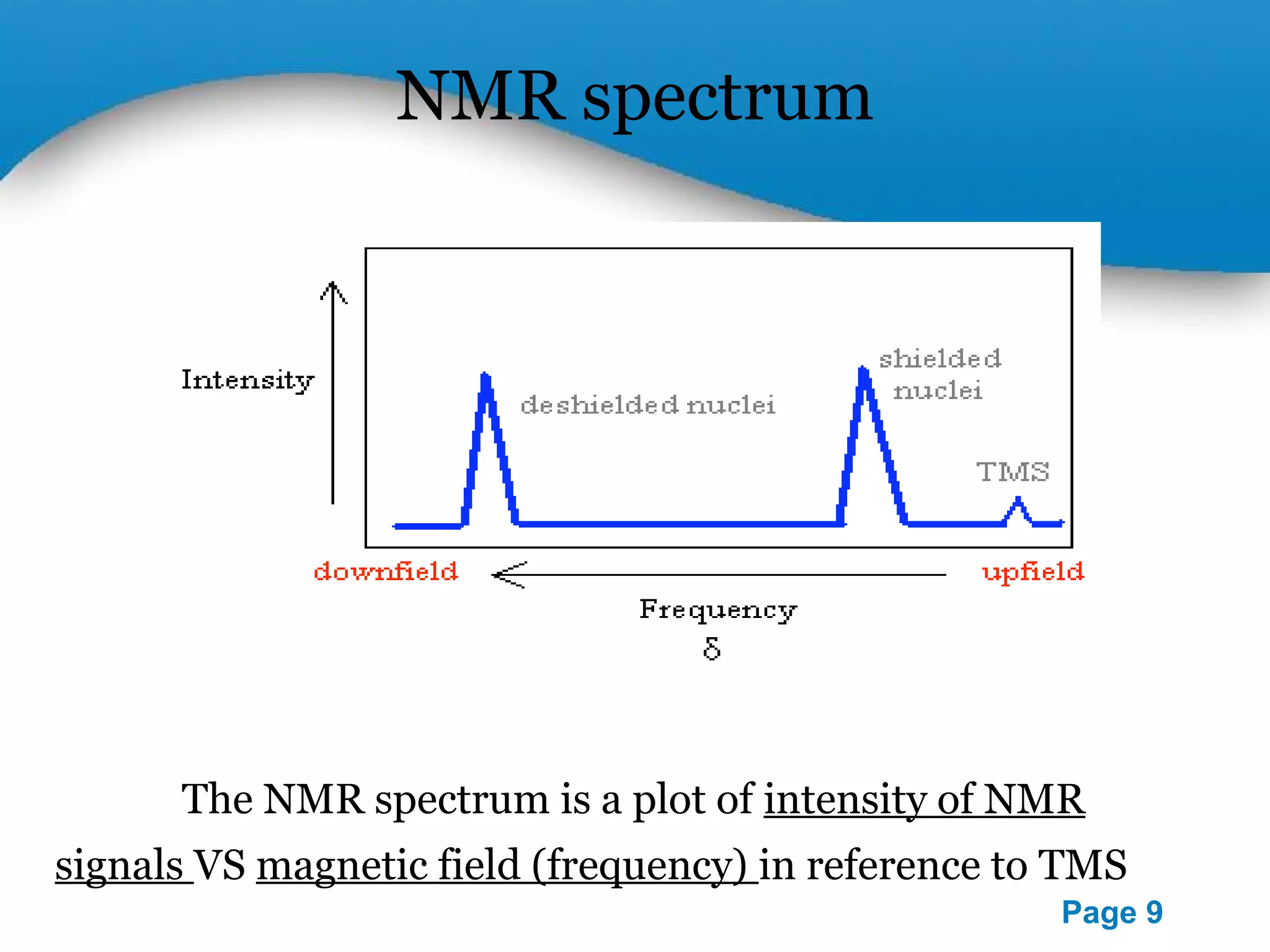
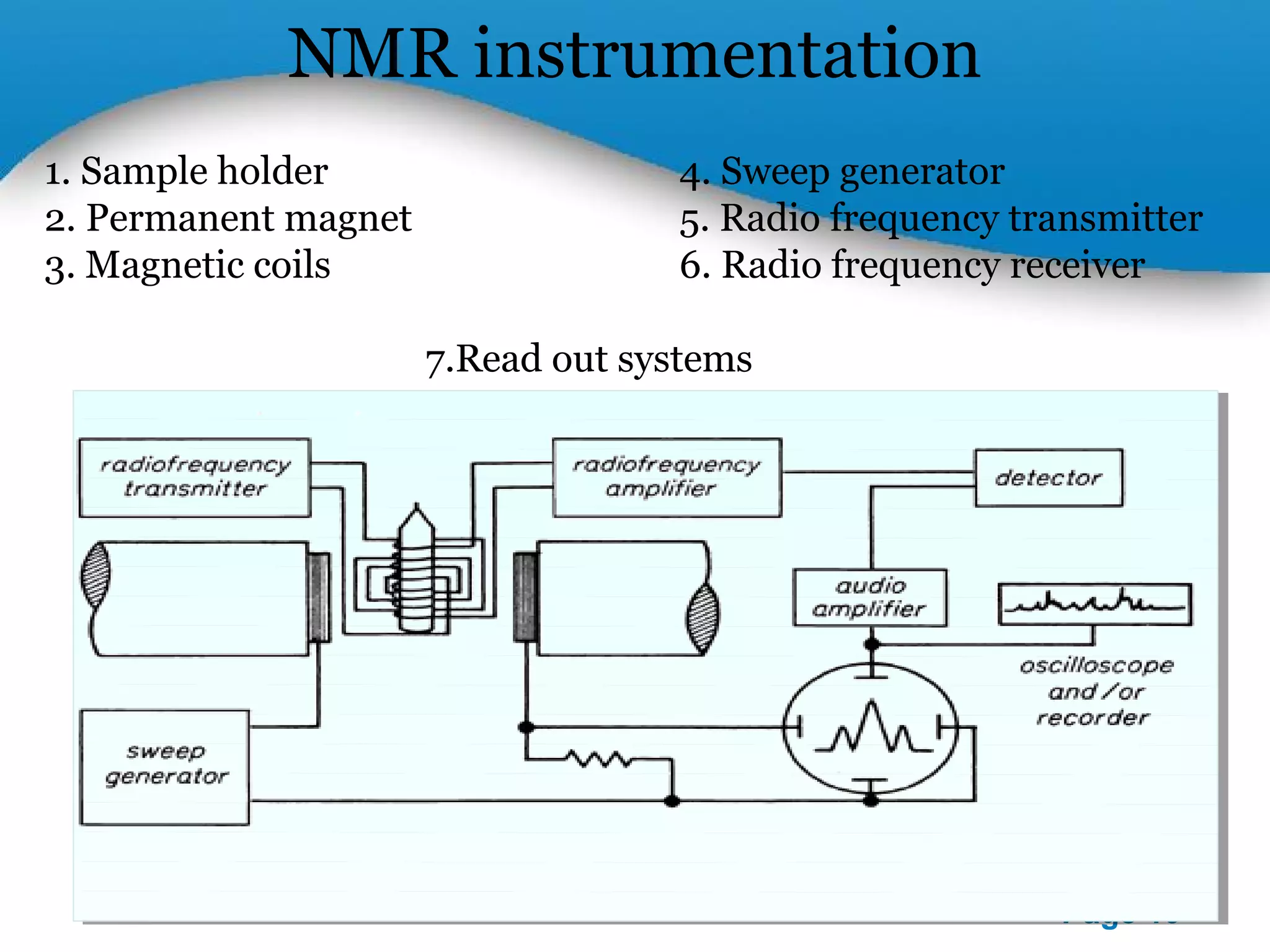
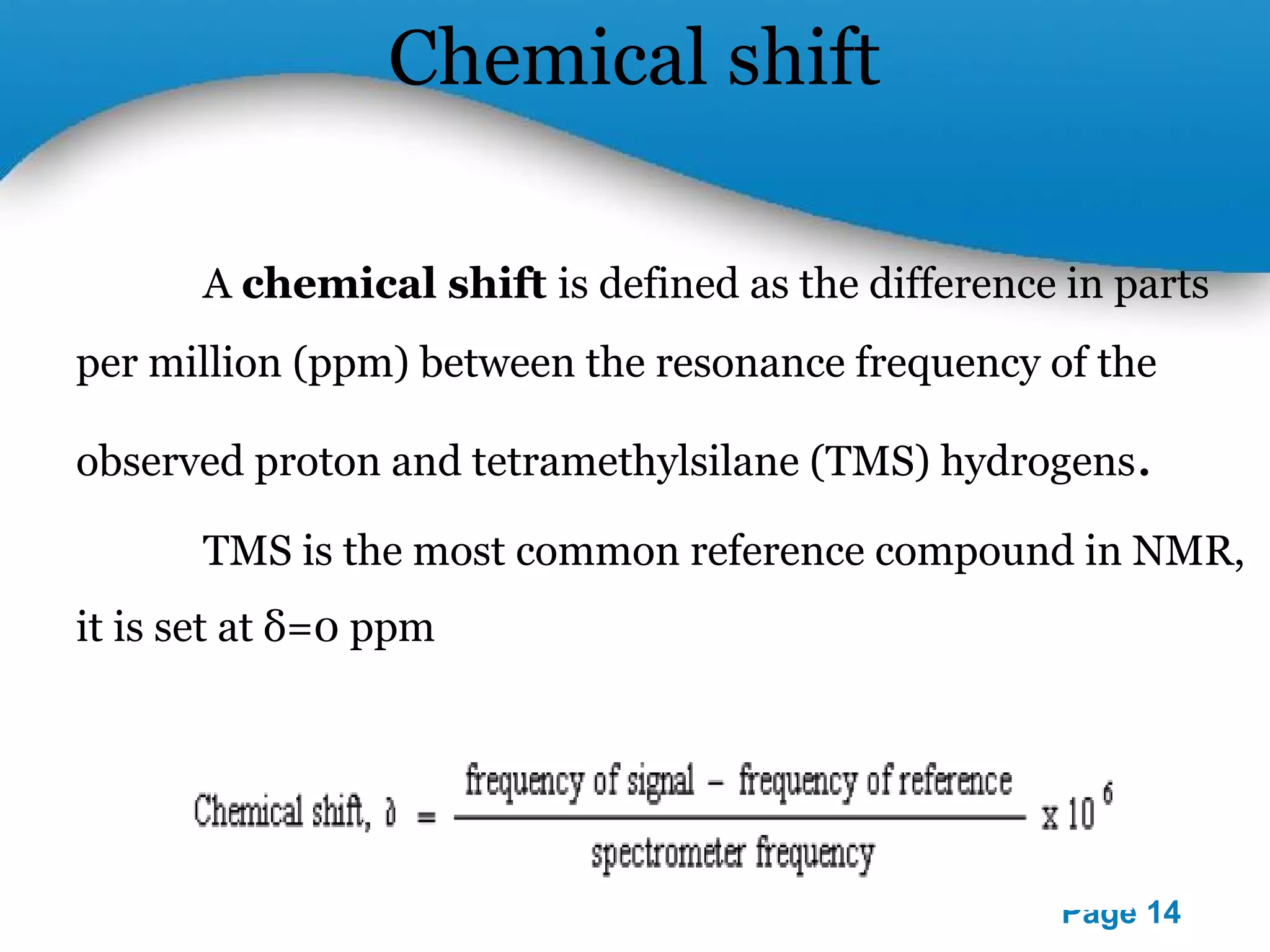
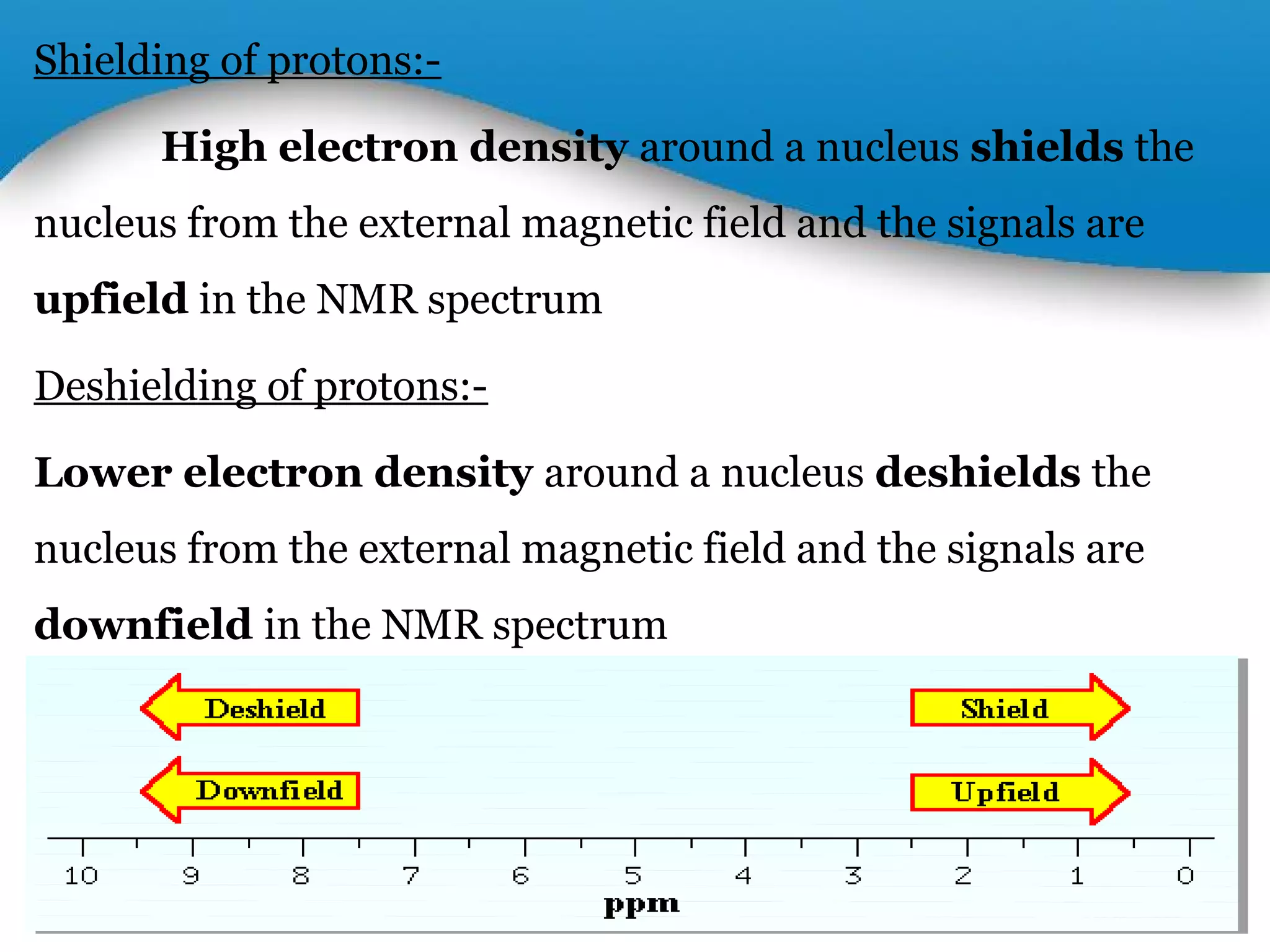
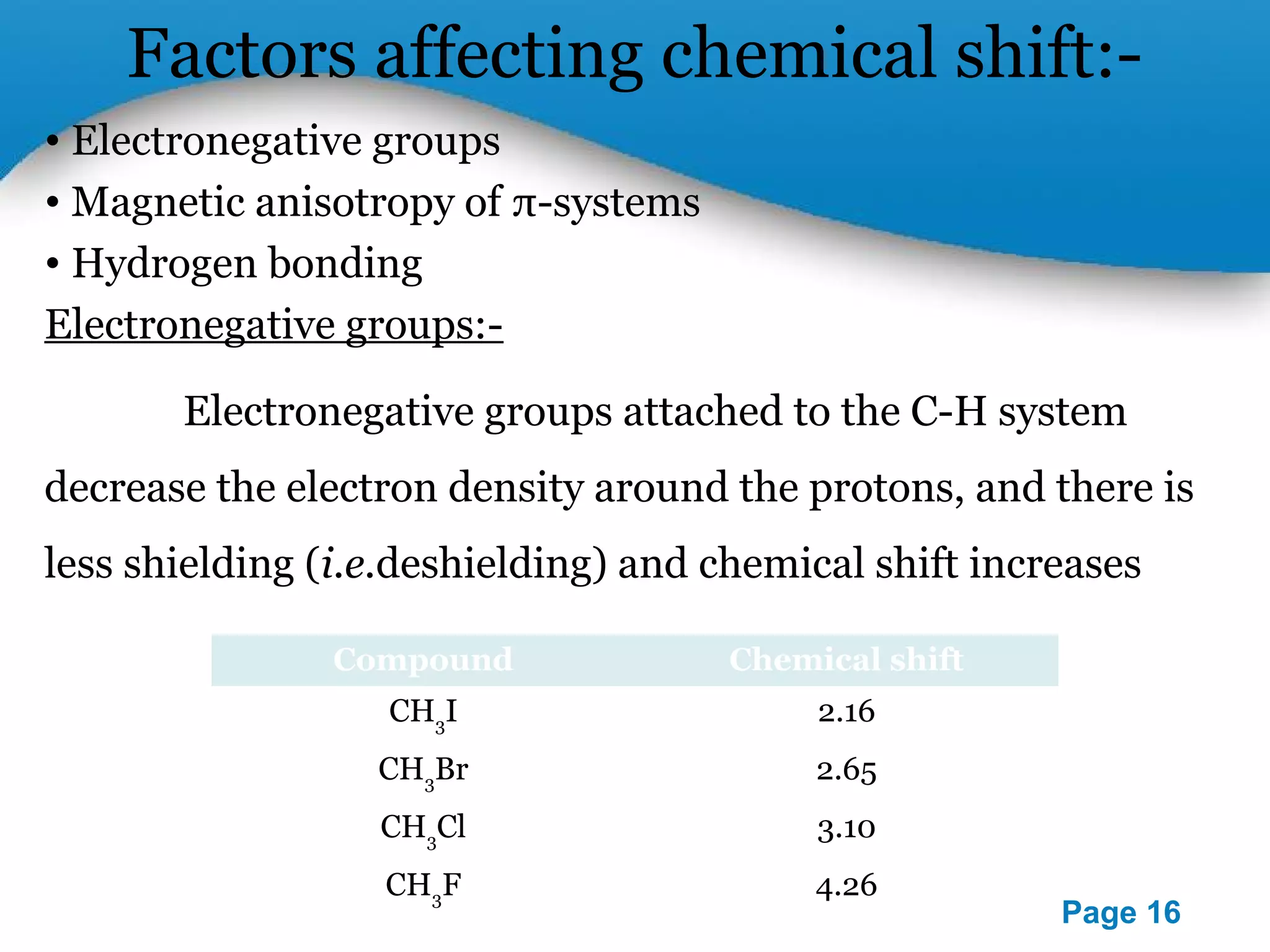
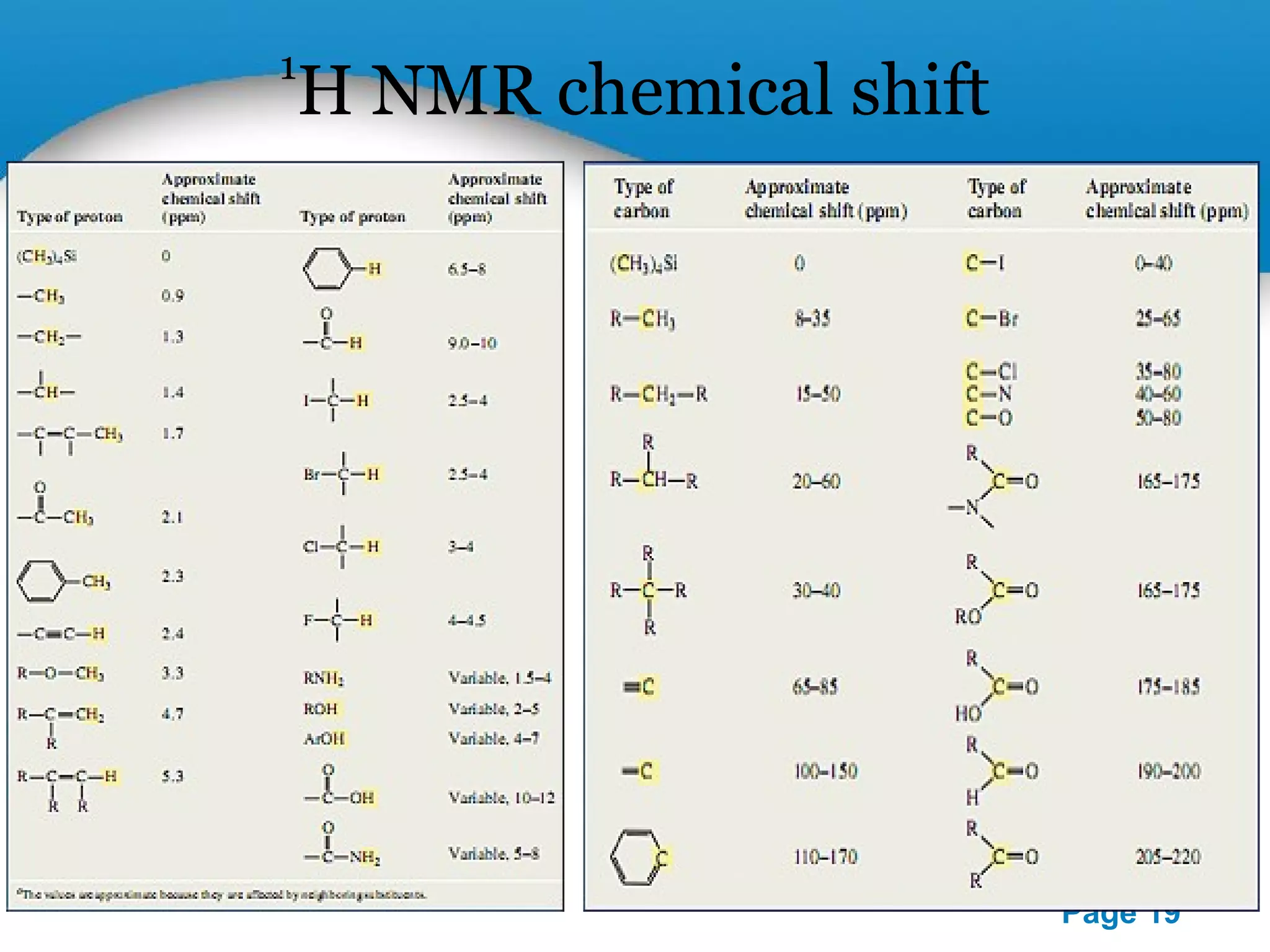
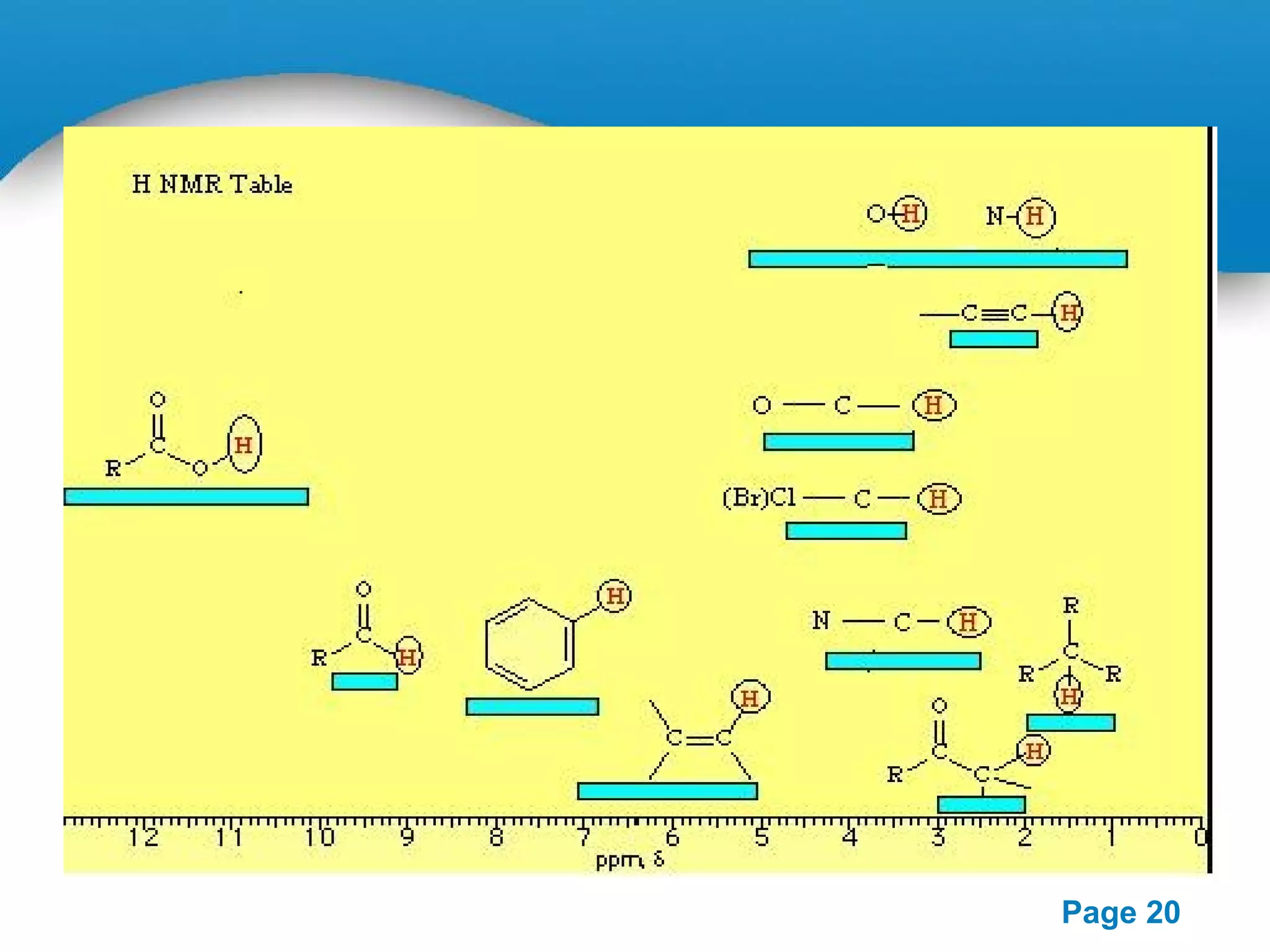
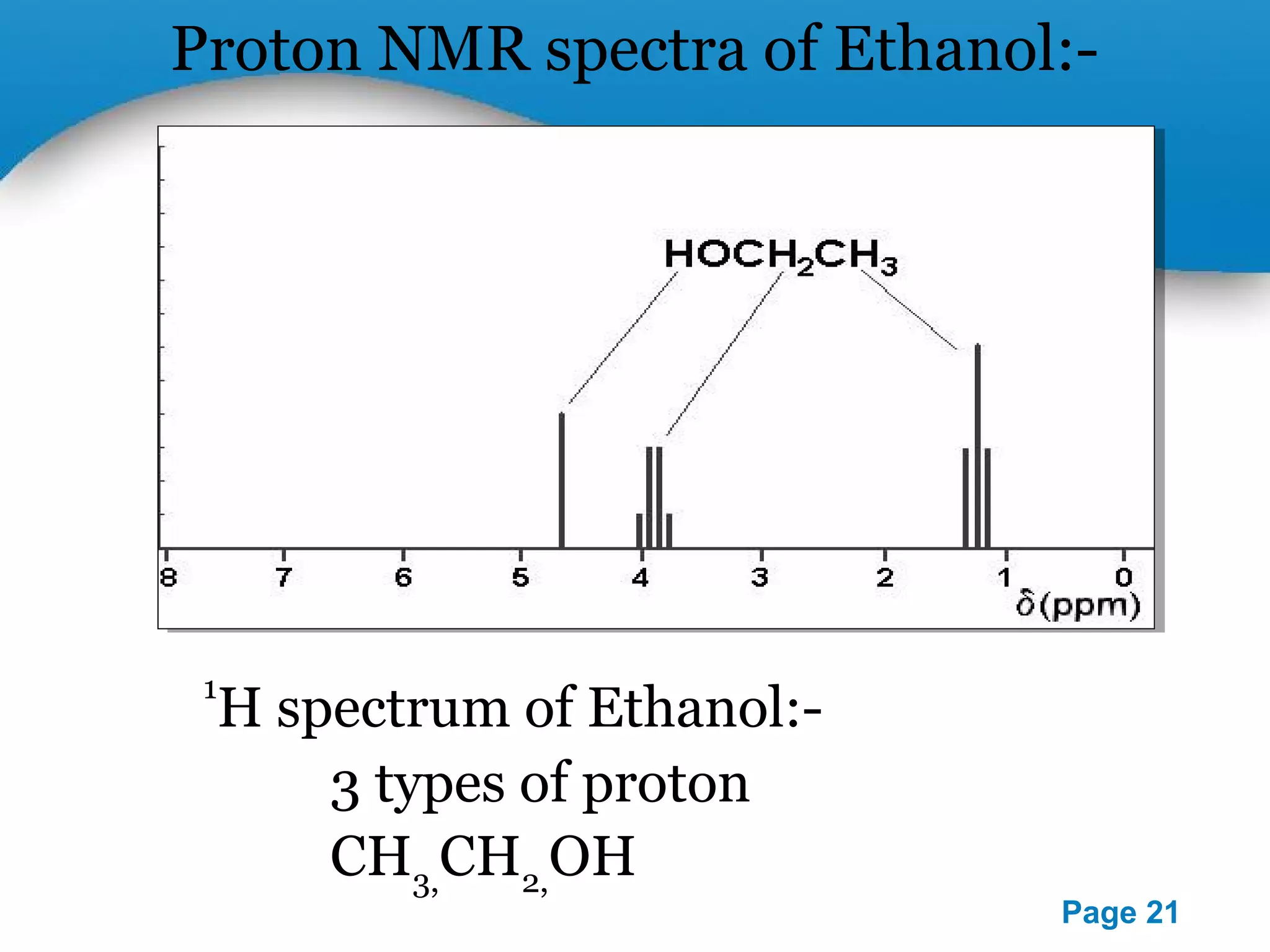
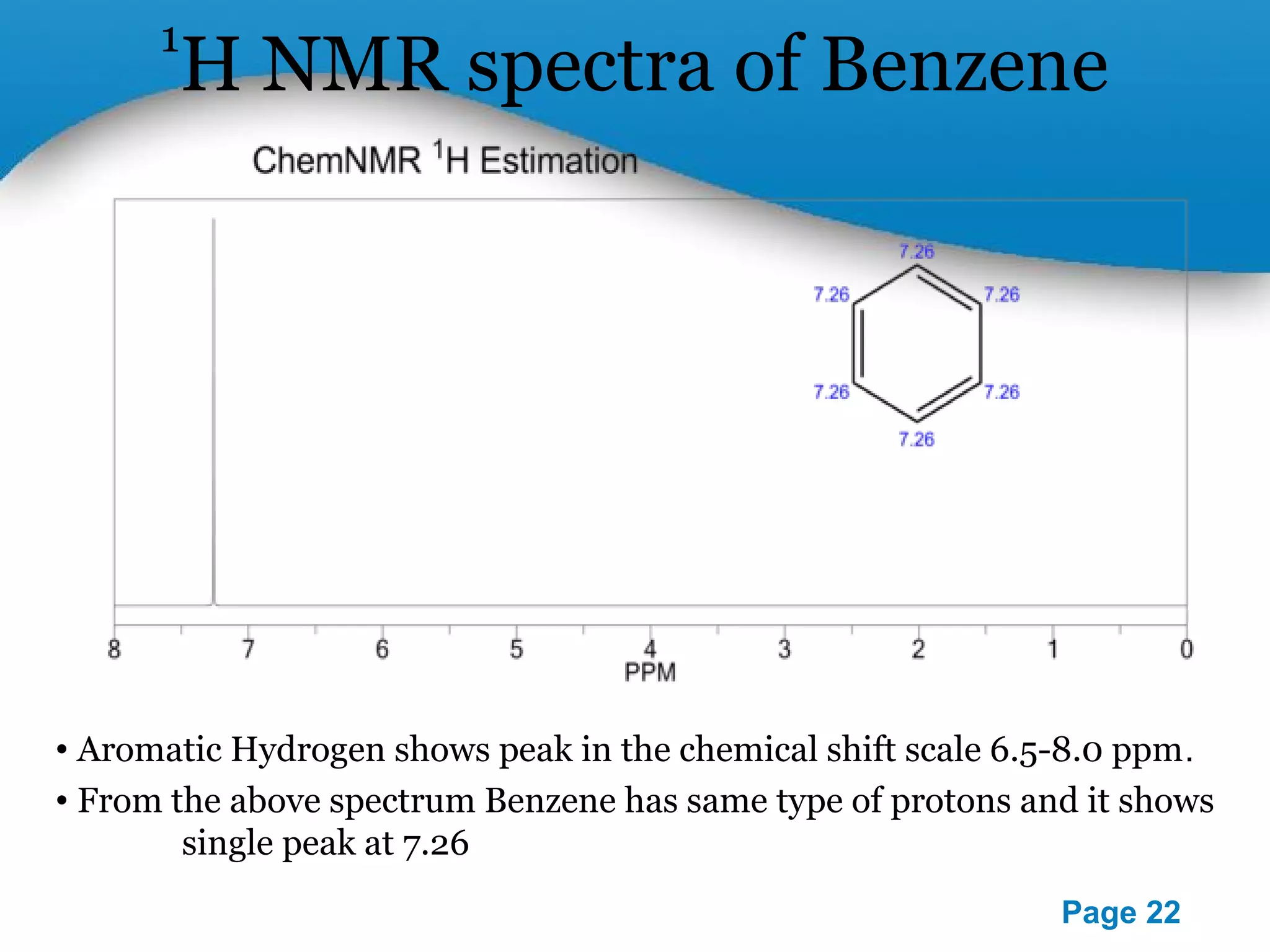
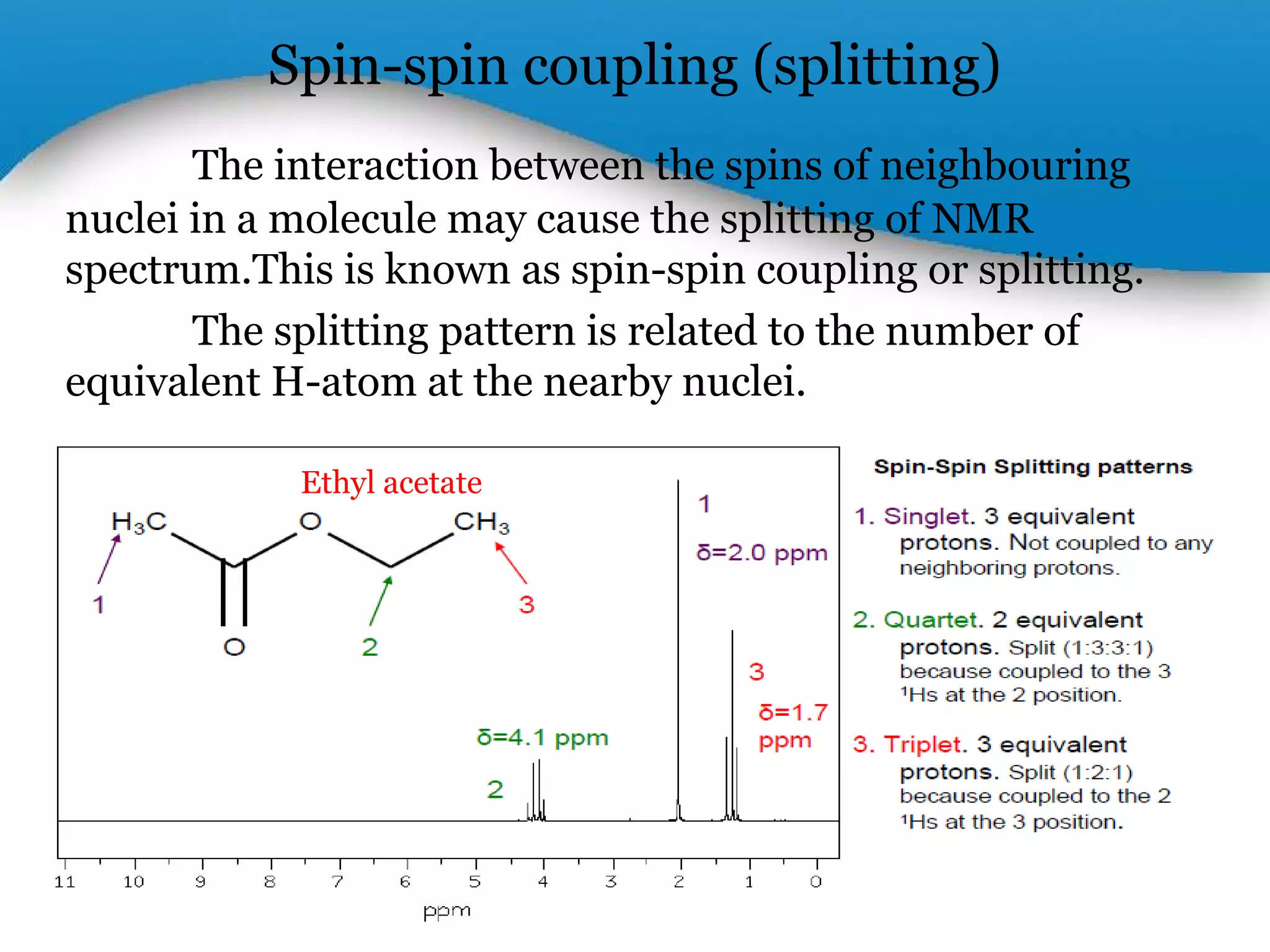
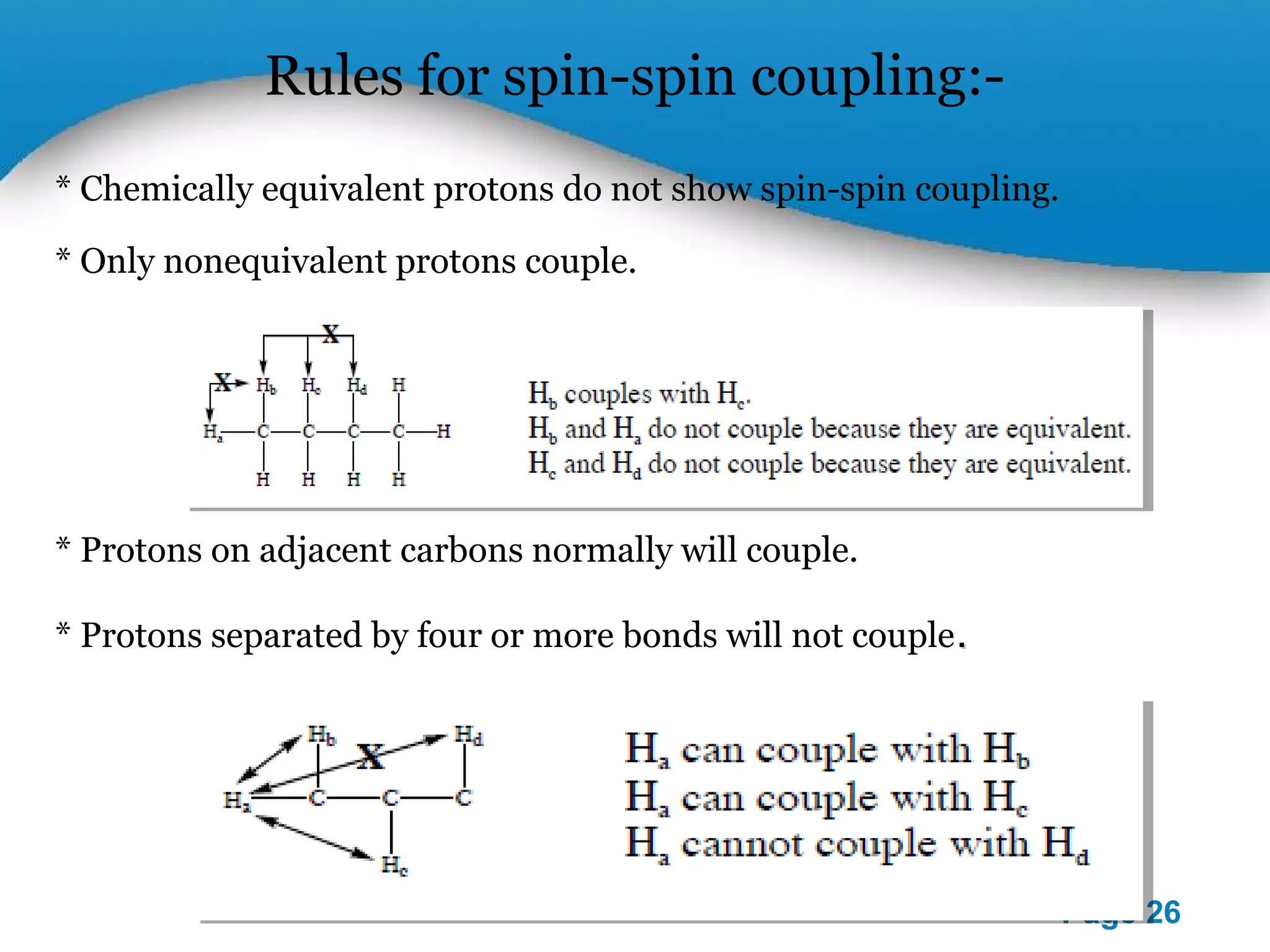
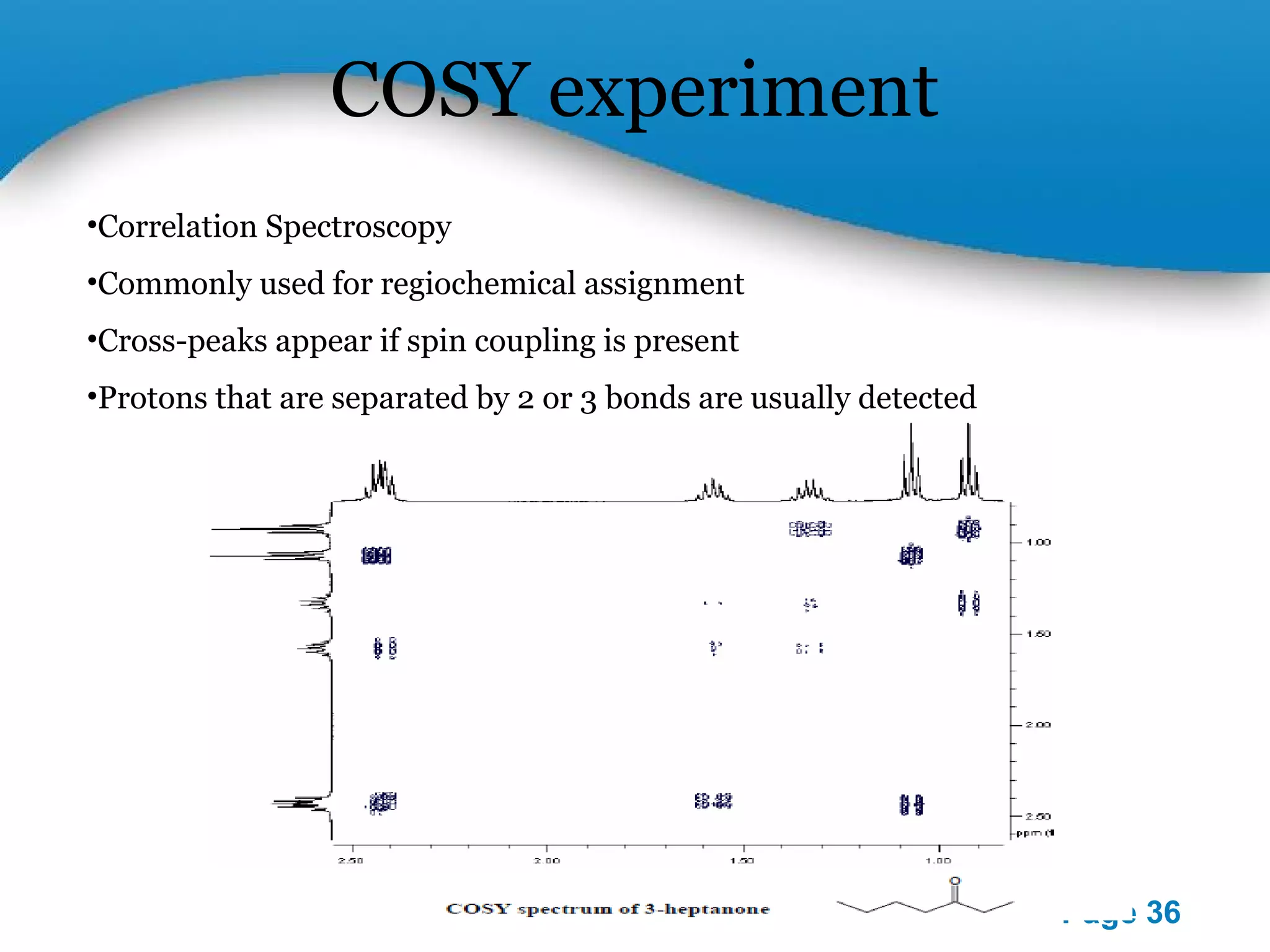
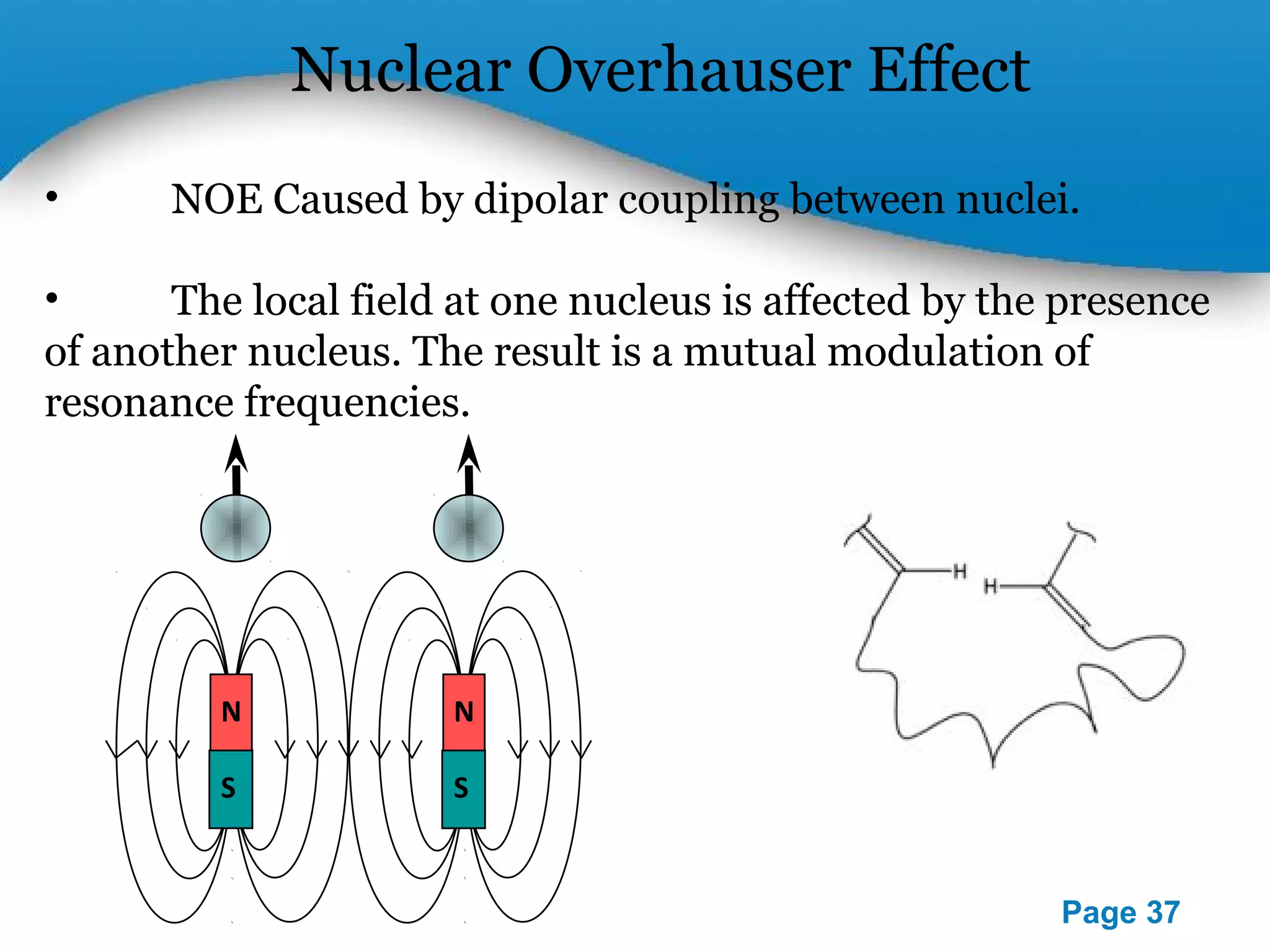
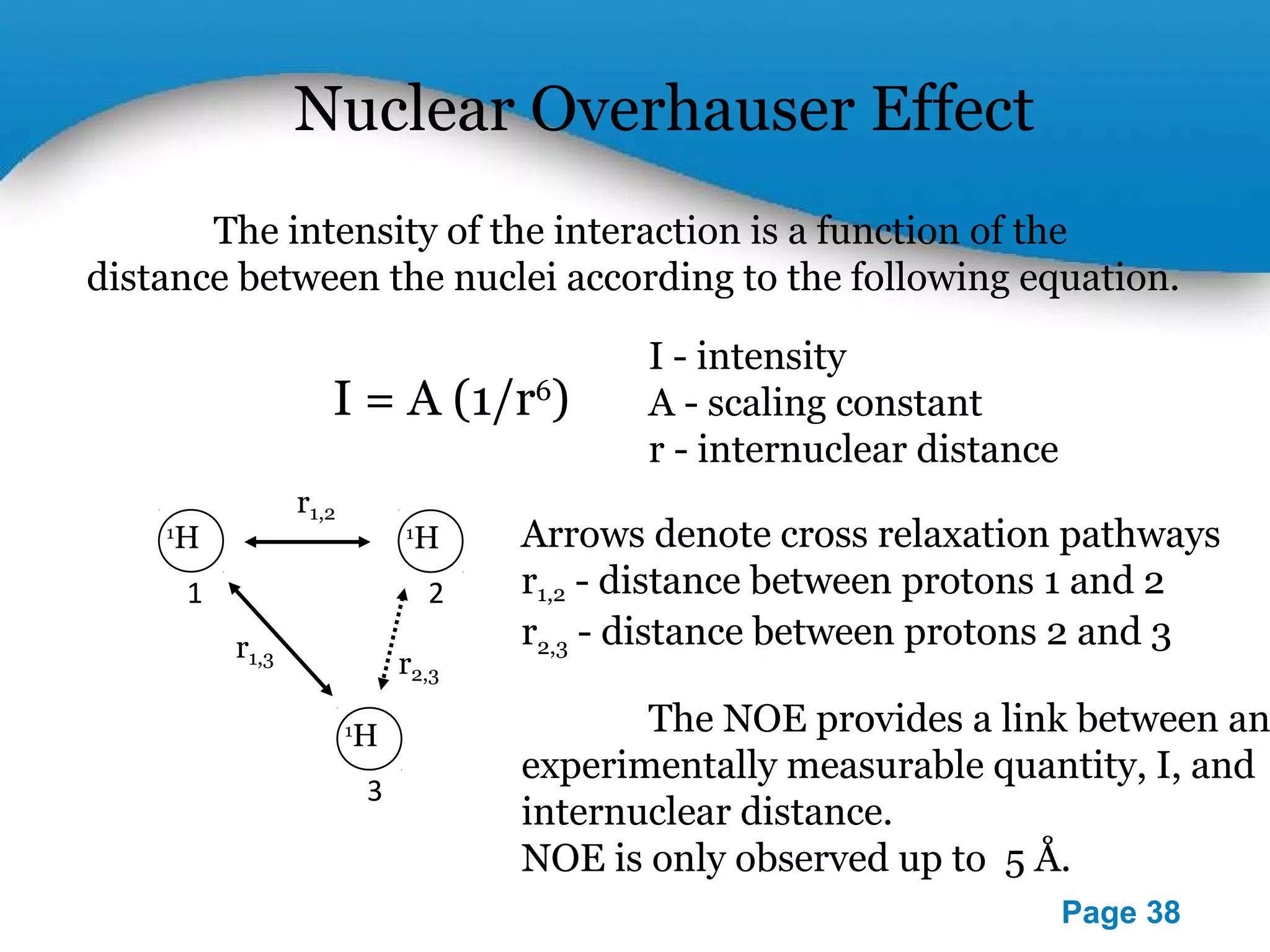
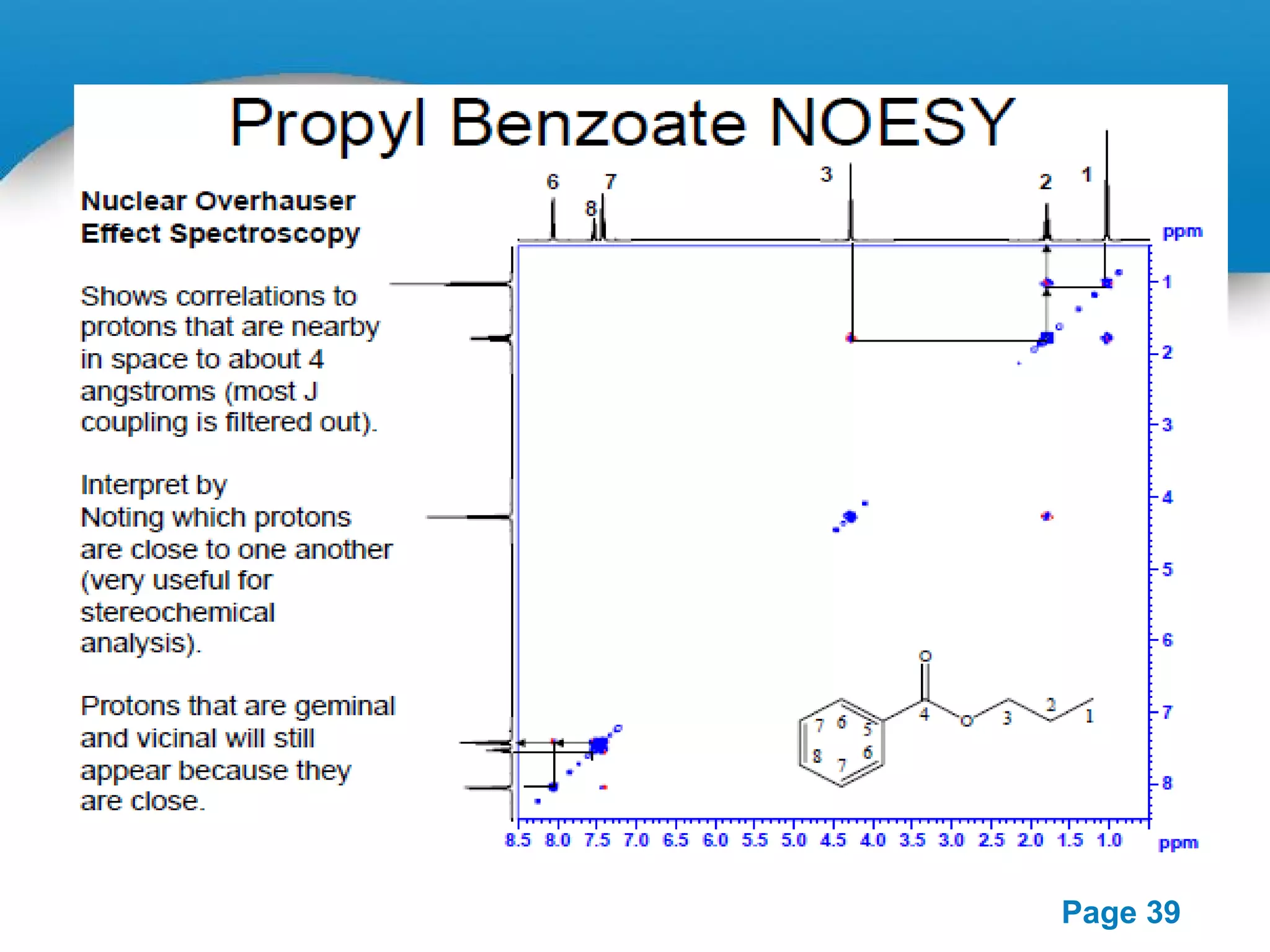
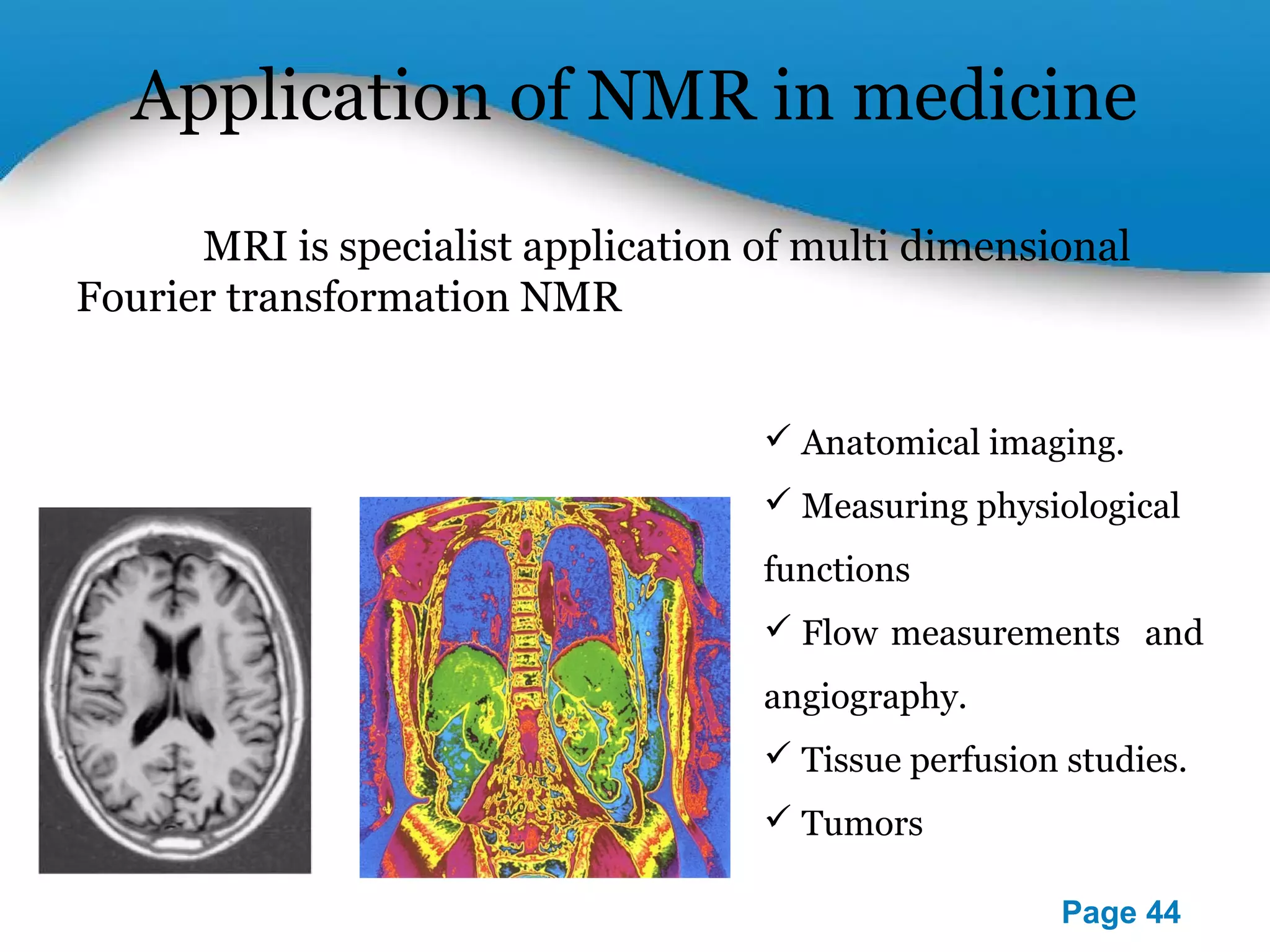
This document outlines a PowerPoint presentation on nuclear magnetic resonance (NMR) spectroscopy. It covers the fundamentals of NMR including spin-spin coupling, instrumentation, solvents, chemical shifts, and 2D NMR techniques. Applications discussed include structure elucidation of organic compounds and biomolecules, as well as clinical uses such as MRI. Specific NMR experiments summarized are COSY, NOESY, and HETCOR.