Report
Share
Download to read offline
More Related Content
Viewers also liked
Viewers also liked (6)
Gr12 apr15 rate law
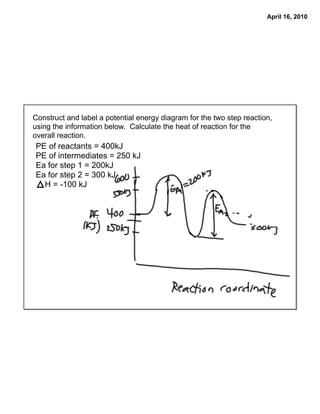
- 1. April 16, 2010 Construct and label a potential energy diagram for the two step reaction, using the information below. Calculate the heat of reaction for the overall reaction. PE of reactants = 400kJ PE of intermediates = 250 kJ Ea for step 1 = 200kJ Ea for step 2 = 300 kJ H = -100 kJ