Lecture 18.3- Solubility
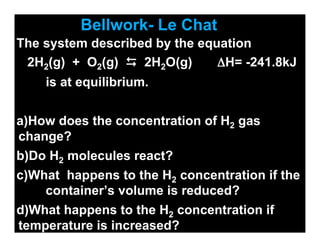
- 1. Bellwork- Le Chat The system described by the equation 2H2(g) + O2(g) 2H2O(g) ΔH= -241.8kJ is at equilibrium. a)How does the concentration of H2 gas change? b)Do H2 molecules react? c)What happens to the H2 concentration if the container’s volume is reduced? d)What happens to the H2 concentration if temperature is increased?
- 2. Solubility The final concentration of ions in solution is different for every substance. Insoluble very few ions in solution
- 3. The solubility product constant (Ksp), equals the product of the concentrations of the ions, each raised to a power equal to the coefficient of the ion in the dissociation equation. Example- PbCl2(s) Pb2+(aq) + 2Cl-(aq) Ksp= [Pb2+][Cl-]2= 1.7x10-5 The smaller the solubility product constant (Ksp), the lower the solubility of the compound.
- 4. 18.3
- 5. 18.3
- 6. 18.3 Silver chloride is slightly soluble in water.
- 7. 18.3 Scale, formed by the precipitation of slightly soluble salts, builds up around faucets.
- 8. 18.3
- 9. 18.3
- 10. 18.3
- 11. 18.3
- 12. for Sample Problem 18.3
- 13. 18.3 PbCl2(s) Pb2+(aq) + 2Cl-(aq) Ksp= [Pb2+][Cl-]2= 1.7x10-5 If the product of the concentrations is greater than the Ksp, a precipitate will form.
- 14. 18.3 The Common Ion Effect A common ion is an ion that is found in both salts in a solution. Common ion effect- solubility lowers when a common ion is added
- 15. The Common Ion Effect A saturated When a few drops solution of of lead nitrate are lead(II) added to the chromate is solution, more pale yellow. lead(II) chromate precipitates.
- 16. The Common Ion Effect Precipitation occurs until Ksp is again satisfied Ksp = [Pb2+][CrO42+] A When This constant this must goes go up↑ down↓
- 17. 18.3 The Common Ion Effect Le Chatelier’s Principle PbCrO4(s) Pb2+(aq) + CrO42+(aq) More Increase solid is Shift left to Pb2+ remove Pb2+ formed
- 18. 18.3 Section Quiz. 1. What is the concentration of a saturated solution of silver sulfide? The Ksp of Ag2S is 8.0 × 10-51. a. 2.0 × 10-17M b. 8.9 × 10-26M c. 8.9 × 10-25M d. 2.0 × 1017M
- 19. 18.3 Section Quiz. 2. Adding which of these solutions to a saturated solution of BaSO4 will cause the solubility of BaSO4 to decrease? I. BaCl2(aq) II. Na2SO4 (aq) a. (I) only b. (II) only c. (I) and (II) d. neither solution
- 20. 18.3 Section Quiz. 3. The Ksp of AgBr is 5.0 × 10-13. When 7.1 × 10-6 mol/L solutions of NaBr(aq) and AgNO3(aq) are mixed, we would expect a. no precipitate to form. b. a definite precipitation reaction. c. no reaction. d. a saturated solution but no visible precipitation.
- 21. 18.3 Section Quiz. 4. After the common ion effect causes a precipitate to form in a solution, a. the solution will no longer be saturated. b. the solution will again be saturated. c. the solution will be supersaturated. d. there will be no solute left in the solution.