11b autonaumic ns
- 1. Extracellular Fluid—The “Internal Environment”
- 2. Homeostasis + -
- 4. Anatomical organization of the somatic and autonomic motor pathways
- 6. Anatomical organization of the sympathetic Nerves
- 10. Autonomic Regulation of Cardiovascular Function
- 12. Autonomic Regulation of the Bladder
- 13. Autonomic Regulation of Sexual Function
- 15. Higher Control of the Autonomic Nervous System
- 16. The Hypothalamus
- 17. Hypothalamus
- 18. Hypothalamic afferents from Limbic System
- 19. Hypothalamic afferents from Cerebral Cortex
- 20. Hypothalamic efferents to thalamus and mammillary body
- 21. Hypothalamic efferents to Pituitary gland and Frontal Lobe
- 22. Hypothalamic control of Endocrine system
- 24. Homeostatic processes can be analyzed in terms of control systems
- 26. Feeding Behavior Is Regulated by a Variety of Mechanisms
- 27. Feeding Behavior
- 29. Food Intake Is Controlled by Short-Term and Long-Term Cues
- 30. Hypothalamus and Energy Homeostasis
- 31. Drinking Is Regulated by Tissue Osmolality and Vascular Volume
- 32. Thanks
Editor's Notes
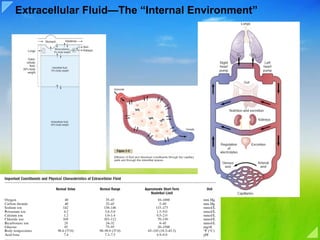
- Extracellular Fluid—The “Internal Environment” About 60 per cent of the adult human body is fluid, mainly a water solution of ions and other substances. Although most of this fluid is inside the cells and is called intracellular fluid, about one third is in the spaces outside the cells and is called extracellular fluid. This extracellular fluid is in constant motion throughout the body. It is transported rapidly in the circulating blood and then mixed between the blood and the tissue fluids by diffusion through the capillary walls. In the extracellular fluid are the ions and nutrients needed by the cells to maintain cell life. Thus, all cells live in essentially the same environment—the extracellular fluid. For this reason, the extracellular fluid is also called the internal environment of the body, or the milieu intérieur, a term introduced more than 100 years ago by the great 19th-century French physiologist Claude Bernard. Cells are capable of living, growing, and performing their special functions as long as the proper concentrations of oxygen, glucose, different ions, amino acids, fatty substances, and other constituents are available in this internal environment. Differences Between Extracellular and Intracellular Fluids. The extracellular fluid contains large amounts of sodium, chloride, and bicarbonate ions plus nutrients for the cells, such as oxygen, glucose, fatty acids, and amino acids. It also contains carbon dioxide that is being transported from the cells to the lungs to be excreted, plus other cellular waste products that are being transported to the kidneys for excretion. The intracellular fluid differs significantly from the extracellular fluid; specifically, it contains large amounts of potassium, magnesium, and phosphate ions instead of the sodium and chloride ions found in the extracellular fluid. Special mechanisms for transporting ions through the cell membranes maintain the ion concentration differences between the extracellular and intracellular fluids. Extracellular Fluid Transport and Mixing System—The Blood Circulatory System Extracellular fluid is transported through all parts of the body in two stages. The first stage is movement of blood through the body in the blood vessels, and the second is movement of fluid between the blood capillaries and the intercellular spaces between the tissue cells. Figure 1–1 shows the overall circulation of blood. All the blood in the circulation traverses the entire circulatory circuit an average of once each minute when the body is at rest and as many as six times each minute when a person is extremely active. As blood passes through the blood capillaries, continual exchange of extracellular fluid also occurs between the plasma portion of the blood and the interstitial fluid that fills the intercellular spaces. This process is shown in Figure 1–2. The walls of the capillaries are permeable to most molecules in the plasma of the blood, with the exception of the large plasma protein molecules. Therefore, large amounts of fluid and its dissolved constituents diffuse back and forth between the blood and the tissue spaces, as shown by the arrows. This process of diffusion is caused by kinetic motion of the molecules in both the plasma and the interstitial fluid. That is, the fluid and dissolved molecules are continually moving and bouncing in all directions within the plasma and the fluid in the intercellular spaces, and also through the capillary pores. Few cells are located more than 50 micrometers from a capillary, which ensures diffusion of almost any substance from the capillary to the cell within a few seconds.Thus, the extracellular fluid everywhere in the body—both that of the plasma and that of the interstitial fluid—is continually being mixed, thereby maintaining almost complete homogeneity of the extracellular fluid throughout the body. Origin of Nutrients in the Extracellular Fluid Respiratory System. Figure 1–1 shows that each time the blood passes through the body, it also flows through the lungs. The blood picks up oxygen in the alveoli, thus acquiring the oxygen needed by the cells. The membrane between the alveoli and the lumen of the pulmonary capillaries, the alveolar membrane, is only 0.4 to 2.0 micrometers thick, and oxygen diffuses by molecular motion through the pores of this membrane into the blood in the same manner that water and ions diffuse through walls of the tissue capillaries. Gastrointestinal Tract. A large portion of the blood pumped by the heart also passes through the walls of the gastrointestinal tract. Here different dissolved nutrients, including carbohydrates, fatty acids, and amino acids, are absorbed from the ingested food into the extracellular fluid of the blood. Liver and Other Organs That Perform Primarily Metabolic Functions. Not all substances absorbed from the gastrointestinal tract can be used in their absorbed form by the cells. The liver changes the chemical compositions of many of these substances to more usable forms, and other tissues of the body—fat cells, gastrointestinal mucosa, kidneys, and endocrine glands—help modify the absorbed substances or store them until they are needed. Musculoskeletal System. Sometimes the question is asked, How does the musculoskeletal system fit into the homeostatic functions of the body? The answer is obvious and simple: Were it not for the muscles, the body could not move to the appropriate place at the appropriate time to obtain the foods required for nutrition. The musculoskeletal system also provides motility for protection against adverse surroundings, without which the entire body, along with its homeostatic mechanisms, could be destroyed instantaneously. Removal of Metabolic End Products Removal of Carbon Dioxide by the Lungs. At the same time that blood picks up oxygen in the lungs, carbon dioxide is released from the blood into the lung alveoli; the respiratory movement of air into and out of the lungs carries the carbon dioxide to the atmosphere. Carbon dioxide is the most abundant of all the end products of metabolism. Kidneys. Passage of the blood through the kidneys removes from the plasma most of the other substances besides carbon dioxide that are not needed by the cells. These substances include different end products of cellular metabolism, such as urea and uric acid; they also include excesses of ions and water from the food that might have accumulated in the extracellular fluid. The kidneys perform their function by first filtering large quantities of plasma through the glomeruli into the tubules and then reabsorbing into the blood those substances needed by the body, such as glucose, amino acids, appropriate amounts of water, and many of the ions. Most of the other substances that are not needed by the body, especially the metabolic end products such as urea, are reabsorbed poorly and pass through the renal tubules into the urine.
- The concept of homeostasis was first articulated by the French scientist Claude Bernard (1813-1878) in his studies of the maintenance of stability in the "milieu interior." He said, "All the vital mechanisms, varied as they are, have only one object, that of preserving constant the conditions of life in the internal environment" (from Leçons sur les Phénonèmes de la Vie Commune aux Animaux et aux Végétaux , 1879). The term itself was coined by American physiologist Walter Cannon, author of The Wisdom of the Body (1932). The word comes from the Greek homoios (same, like, resembling) and stasis (to stand, posture). a schematic of homeostasis. Changes in the environment are transduced to cause a change in the level of a regulated substance. This change is detected through measurement and comparison with a coded set-point value. Disparities between the measured value and the set-point value regulate a response mechanism that directly or indirectly influences effector systems at the exterior–interior interface. Homeostatic systems often require fuel, other support mechanisms and interact with other systems. What is Homeostasis? Homeostasis in a general sense refers to stability, balance or equilibrium. Maintaining a stable internal environment requires constant monitoring and adjustments as conditions change. This adjusting of physiological systems within the body is called homeostatic regulation. Homeostatic regulation involves three parts or mechanisms: 1) the receptor , 2) the control center and 3) the effector . The receptor receives information that something in the environment is changing. The control center or integration center receives and processes information from the receptor . And lastly, the effector responds to the commands of the control center by either opposing or enhancing the stimulus. A metaphor to help us understand this process is the operation of a thermostat. The thermostat monitors and controls room temperature. The thermostat is set at a certain temperature that is considered ideal, the set point . The function of the thermostat is to keep the temperature in the room within a few degrees of the set point . If the room is colder than the set point , the thermostat receives information from the thermometer (the receptor ) that it is too cold. The effectors within the thermostat then will turn on the heat to warm up the room. When the room temperature reaches the set point , the receptor receives the information, and the thermostat "tells" the heater to turn off. This also works when it is too hot in the room. The thermostat receives the information and turns on the air conditioner. When the set point temperature is reached, the thermostat turns off the air conditioner. Our bodies control body temperature in a similar way. The brain is the control center, the receptor is our body's temperature sensors, and the effector is our blood vessels and sweat glands in our skin. When we feel heat, the temperature sensors in our skin send the message to our brain. Our brain then sends the message to the sweat glands to increase sweating and increase blood flow to our skin. When we feel cold, the opposite happens. Our brain sends a message to our sweat glands to decrease sweating, decrease blood flow, and begin shivering. This is an ongoing process that continually works to restore and maintain homeostasis. Because the internal and external environment of the body are constantly changing and adjustments must be made continuously to stay at or near the set point, homeostasis can be thought of as a dynamic equilibrium. Positive and Negative Feedback When a change of variable occurs, there are two main types of feedback to which the system reacts: • Negative feedback : a reaction in which the system responds in such a way as to reverse the direction of change. Since this tends to keep things constant, it allows the maintenance of homeostasis. For instance, when the concentration of carbon dioxide in the human body increases, the lungs are signaled to increase their activity and expel more carbon dioxide. Thermoregulation is another example of negative feedback. When body temperature rises (or falls), receptors in the skin and the hypothalamus sense a change, triggering a command from the brain. This command, in turn, effects the correct response, in this case a decrease in body temperature. • Home Heating System Vs. Negative Feedback: When you are home, you set your thermostat to a desired temperature. Let's say today you set it at 70 degrees. The thermometer in the thermostat waits to sense a temperature change either too high above or too far below the 70 degree set point. When this change happens the thermometer will send a message to the "Control Center", or thermostat, Which in turn will then send a message to the furnace to either shut off if the temperature is too high or kick back on if the temperature is too low. In the home-heating example the air temperature is the "NEGATIVE FEEDBACK." When the Control Center receives negative feedback it triggers a chain reaction in order to maintain room temperature. • Positive feedback : a response is to amplify the change in the variable. This has a destabilizing effect, so does not result in homeostasis. Positive feedback is less common in naturally occurring systems than negative feedback, but it has its applications. For example, in nerves, a threshold electric potential triggers the generation of a much larger action potential. Blood clotting and events in childbirth are other types of positive feedback. ' *Harmful Positive Feedback' Although Positive Feedback is needed within Homeostasis it also can be harmful at times. When you have a high fever it causes a metabolic change that can push the fever higher and higher. In rare occurrences the the body temperature reaches 113 degrees the cellular proteins stop working and the metabolism stops, resulting in death. Summary: Sustainable systems require combinations of both kinds of feedback. Generally with the recognition of divergence from the homeostatic condition, positive feedbacks are called into play, whereas once the homeostatic condition is approached, negative feedback is used for "fine tuning" responses. This creates a situation of "metastability," in which homeostatic conditions are maintained within fixed limits, but once these limits are exceeded, the system can shift wildly to a wholly new (and possibly less desirable) situation of homeostasis. Homeostatic systems have several properties • They are ultra-stable, meaning the system is capable of testing which way its variables should be adjusted. • Their whole organization (internal, structural, and functional) contributes to the • Physiology is largely a study of processes related to homeostasis. Some of the functions you will learn about in this book are not specifically about homeostasis (e.g. how muscles contract), but in order for all bodily processes to function there must be a suitable internal environment. Homeostasis is, therefore, a fitting framework for the introductory study of physiology. Pathways That Alter Homeostasis A variety of homeostatic mechanisms maintain the internal environment within tolerable limits. Either homeostasis is maintained through a series of control mechanisms, or the body suffers various illnesses or disease. When the cells in your body begin to malfunction, the homeostatic balance becomes disrupted. Eventually this leads to disease or cell malfunction. Disease and cellular malfunction can be caused in two basic ways: either, deficiency (cells not getting all they need) or toxicity (cells being poisoned by things they do not need). When homeostasis is interrupted in your cells, there are pathways to correct or worsen the problem. In addition to the internal control mechanisms, there are external influences based primarily on lifestyle choices and environmental exposures that influence our body's ability to maintain cellular health. • Nutrition: If your diet is lacking in a specific vitamin or mineral your cells will function poorly, possibly resulting in a disease condition. For example, a menstruating woman with inadequate dietary intake of iron will become anemic. Lack of hemoglobin, a molecule that requires iron, will result in reduced oxygen-carrying capacity. In mild cases symptoms may be vague (e.g. fatigue), but if the anemia is severe the body will try to compensate by increasing cardiac output, leading to palpitations and sweatiness, and possibly to heart failure. • Toxins: Any substance that interferes with cellular function, causing cellular malfunction. This is done through a variety of ways; chemical, plant, insecticides, and or bites. A commonly seen example of this is drug overdoses. When a person takes too much of a drug their vital signs begin to waver; either increasing or decreasing, these vital signs can cause problems including coma, brain damage and even death. • Psychological: Your physical health and mental health are inseparable. Our thoughts and emotions cause chemical changes to take place either for better as with meditation, or worse as with stress. • Physical: Physical maintenance is essential for our cells and bodies. Adequate rest, sunlight, and exercise are examples of physical mechanisms for influencing homeostasis. Lack of sleep is related to a number of ailments such as irregular cardiac rhythms, fatigue, anxiety and headaches. • Genetic/Reproductive: Inheriting strengths and weaknesses can be part of our genetic makeup. Genes are sometimes turned off or on due to external factors which we can have some control over, but at other times little can be done to correct or improve genetic diseases. Beginning at the cellular level a variety of diseases come from mutated genes. For example, cancer can be genetically inherited or can be caused due to a mutation from an external source such as radiation or genes altered in a fetus when the mother uses drugs. • Medical: Because of genetic differences some bodies need help in gaining or maintaining homeostasis. Through modern medicine our bodies can be given different aids -from anti-bodies to help fight infections or chemotherapy to kill harmful cancer cells. Traditional and alternative medical practices have many benefits, but the potential for harmful effects is also present. Whether by nosocomial infections, or wrong dosage of medication, homeostasis can be altered by that which is trying to fix it. Trial and error with medications can cause potential harmful reactions and possibly death if not caught soon enough. The factors listed above all have their effects at the cellular level, whether harmful or beneficial. Inadequate beneficial pathways (deficiency) will almost always result in a harmful waiver in homeostasis. Too much toxicity also causes homeostatic imbalance, resulting in cellular malfunction. By removing negative health influences, and providing adequate positive health influences, your body is better able to self-regulate and self-repair, thus maintaining homeostasis. Control Systems of the Body The human body has thousands of control systems in it. The most intricate of these are the genetic control systems that operate in all cells to help control intracellular function as well as extracellular function. This subject is discussed in Chapter 3. Many other control systems operate within the organs to control functions of the individual parts of the organs; others operate throughout the entire body to control the interrelations between the organs. For instance, the respiratory system, operating in association with the nervous system, regulates the concentration of carbon dioxide in the extracellular fluid. The liver and pancreas regulate the concentration of glucose in the extracellular fluid, and the kidneys regulate concentrations of hydrogen, sodium, potassium, phosphate, and other ions in the extracellular fluid. Examples of Control Mechanisms Regulation of Oxygen and Carbon Dioxide Concentrations in the Extracellular Fluid. Because oxygen is one of the major substances required for chemical reactions in the cells, it is fortunate that the body has a special control mechanism to maintain an almost exact and constant oxygen concentration in the extracellular fluid. This mechanism depends principally on the chemical characteristics of hemoglobin, which is present in all red blood cells. Hemoglobin combines with oxygen as the blood passes through the lungs. Then, as the blood passes through the tissue capillaries, hemoglobin, because of its own strong chemical affinity for oxygen, does not release oxygen into the tissue fluid if too much oxygen is already there. But if the oxygen concentration in the tissue fluid is too low, sufficient oxygen is released to re-establish an adequate concentration. Thus, regulation of oxygen concentration in the tissues is vested principally in the chemical characteristics of hemoglobin itself. This regulation is called the oxygen-buffering function of hemoglobin. Carbon dioxide concentration in the extracellular fluid is regulated in a much different way. Carbon dioxide is a major end product of the oxidative reactions in cells. If all the carbon dioxide formed in the cells continued to accumulate in the tissue fluids, the mass action of the carbon dioxide itself would soon halt all energy-giving reactions of the cells. Fortunately, a higher than normal carbon dioxide concentration in the blood excites the respiratory center, causing a person to breathe rapidly and deeply. This increases expiration of carbon dioxide and, therefore, removes excess carbon dioxide from the blood and tissue fluids. This process continues until the concentration returns to normal. Regulation of Arterial Blood Pressure. Several systems contribute to the regulation of arterial blood pressure. One of these, the baroreceptor system, is a simple and excellent example of a rapidly acting control mechanism. In the walls of the bifurcation region of the carotid arteries in the neck, and also in the arch of the aorta in the thorax, are many nerve receptors called baroreceptors, which are stimulated by stretch of the arterial wall.When the arterial pressure rises too high, the baroreceptors send barrages of nerve impulses to the medulla of the brain. Here these impulses inhibit the vasomotor center, which in turn decreases the number of impulses transmitted from the vasomotor center through the sympathetic nervous system to the heart and blood vessels. Lack of these impulses causes diminished pumping activity by the heart and also dilation of the peripheral blood vessels, allowing increased blood flow through the vessels. Both of these effects decrease the arterial pressure back toward normal. Conversely, a decrease in arterial pressure below normal relaxes the stretch receptors, allowing the vasomotor center to become more active than usual, thereby causing vasoconstriction and increased heart pumping, and raising arterial pressure back toward normal. Normal Ranges and Physical Characteristics of Important Extracellular Fluid Constituents Table 1–1 lists the more important constituents and physical characteristics of extracellular fluid, along with their normal values, normal ranges, and maximum limits without causing death. Note the narrowness of the normal range for each one. Values outside these ranges are usually caused by illness. Most important are the limits beyond which abnormalities can cause death. For example, an increase in the body temperature of only 11°F (7°C) above normal can lead to a vicious cycle of increasing cellular metabolism that destroys the cells. Note also the narrow range for acid-base balance in the body, with a normal pH value of 7.4 and lethal values only about 0.5 on either side of normal. Another important factor is the potassium ion concentration, because whenever it decreases to less than one third normal, a person is likely to be paralyzed as a result of the nerves’ inability to carry signals. Alternatively, if the potassium ion concentration increases to two or more times normal, the heart muscle is likely to be severely depressed. Also, when the calcium ion concentration falls below about one half of normal, a person is likely to experience tetanic contraction of muscles throughout the body because of the spontaneous generation of excess nerve impulses in the peripheral nerves. When the glucose concentration falls below one half of normal, a person frequently develops extreme mental irritability and sometimes even convulsions. These examples should give one an appreciation for the extreme value and even the necessity of the vast numbers of control systems that keep the body operating in health; in the absence of any one of these controls, serious body malfunction or death can result. Characteristics of Control Systems The aforementioned examples of homeostatic control mechanisms are only a few of the many thousands in the body, all of which have certain characteristics in common. These characteristics are explained in this section. Negative Feedback Nature of Most Control Systems Most control systems of the body act by negative feedback, which can best be explained by reviewing some of the homeostatic control systems mentioned previously. In the regulation of carbon dioxide concentration, a high concentration of carbon dioxide in the extracellular fluid increases pulmonary ventilation. This, in turn, decreases the extracellular fluid carbon dioxide concentration because the lungs expire greater amounts of carbon dioxide from the body. In other words, the high concentration of carbon dioxide initiates events that decrease the concentration toward normal, which is negative to the initiating stimulus. Conversely, if the carbon dioxide concentration falls too low, this causes feedback to increase the concentration. This response also is negative to the initiating stimulus. In the arterial pressure–regulating mechanisms, a high pressure causes a series of reactions that promote a lowered pressure, or a low pressure causes a series of reactions that promote an elevated pressure. In both instances, these effects are negative with respect to the initiating stimulus. Therefore, in general, if some factor becomes excessive or deficient, a control system initiates negative feedback, which consists of a series of changes that return the factor toward a certain mean value, thus maintaining homeostasis. “ Gain” of a Control System. The degree of effectiveness with which a control system maintains constant conditions is determined by the gain of the negative feedback. For instance, let us assume that a large volume of blood is transfused into a person whose baroreceptor pressure control system is not functioning, and the arterial pressure rises from the normal level of 100 mm Hg up to 175 mm Hg. Then, let us assume that the same volume of blood is injected into the same person when the baroreceptor system is functioning, and this time the pressure increases only 25 mm Hg. Thus, the feedback control system has caused a “correction” of –50 mm Hg—that is, from 175 mm Hg to 125 mm Hg. There remains an increase in pressure of +25 mm Hg, called the “error,” which means that the control system is not 100 per cent effective in preventing change. The gain of the system is then calculated by the following formula: Thus, in the baroreceptor system example, the correction is –50 mm Hg and the error persisting is +25 mm Hg. Therefore, the gain of the person’s baroreceptor system for control of arterial pressure is –50 divided by +25, or –2. That is, a disturbance that increases or decreases the arterial pressure does so only one third as much as would occur if this control system were not present. The gains of some other physiologic control systems are much greater than that of the baroreceptor system. For instance, the gain of the system controlling internal body temperature when a person is exposed to moderately cold weather is about –33. Therefore, one can see that the temperature control system is much more effective than the baroreceptor pressure control system. Positive Feedback Can Sometimes Cause Vicious Cycles and Death One might ask the question, Why do essentially all control systems of the body operate by negative feedback rather than positive feedback? If one considers the nature of positive feedback, one immediately sees that positive feedback does not lead to stability but to instability and often death. Figure 1–3 shows an example in which death can ensue from positive feedback. This figure depicts the pumping effectiveness of the heart, showing that the heart of a healthy human being pumps about 5 liters of blood per minute. If the person is suddenly bled 2 liters, the amount of blood in the body is decreased to such a low level that not enough blood is available for the heart to pump effectively. As a result, the arterial pressure falls, and the flow of blood to the heart muscle through the coronary vessels diminishes. This results in weakening of the heart, further diminished pumping, a further decrease in coronary blood flow, and still more weakness of the heart; the cycle repeats itself again and again until death occurs. Note that each cycle in the feedback results in further weakening of the heart. In other words, the initiating stimulus causes more of the same, which is positive feedback. Gain = Correction/Error Positive feedback is better known as a “vicious cycle,” but a mild degree of positive feedback can be overcome by the negative feedback control mechanisms of the body, and the vicious cycle fails to develop. For instance, if the person in the aforementioned example were bled only 1 liter instead of 2 liters, the normal negative feedback mechanisms for controlling cardiac output and arterial pressure would overbalance the positive feedback and the person would recover, as shown by the dashed curve of Figure 1–3. Positive Feedback Can Sometimes Be Useful. In some instances, the body uses positive feedback to its advantage. Blood clotting is an example of a valuable use of positive feedback. When a blood vessel is ruptured and a clot begins to form, multiple enzymes called clotting factors are activated within the clot itself. Some of these enzymes act on other unactivated enzymes of the immediately adjacent blood, thus causing more blood clotting. This process continues until the hole in the vessel is plugged and bleeding no longer occurs. On occasion, this mechanism can get out of hand and cause the formation of unwanted clots. In fact, this is what initiates most acute heart attacks, which are caused by a clot beginning on the inside surface of an atherosclerotic plaque in a coronary artery and then growing until the artery is blocked. Childbirth is another instance in which positive feedback plays a valuable role. When uterine contractions become strong enough for the baby’s head to begin pushing through the cervix, stretch of the cervix sends signals through the uterine muscle back to the body of the uterus, causing even more powerful contractions. Thus, the uterine contractions stretch the cervix, and the cervical stretch causes stronger contractions. When this process becomes powerful enough, the baby is born. If it is not powerful enough, the contractions usually die out, and a few days pass before they begin again. Another important use of positive feedback is for the generation of nerve signals. That is, when the membrane of a nerve fiber is stimulated, this causes slight leakage of sodium ions through sodium channels in the nerve membrane to the fiber’s interior. The sodium ions entering the fiber then change the membrane potential, which in turn causes more opening of channels, more change of potential, still more opening of channels, and so forth. Thus, a slight leak becomes an explosion of sodium entering the interior of the nerve fiber, which creates the nerve action potential. This action potential in turn causes electrical current to flow along both the outside and the inside of the fiber and initiates additional action potentials. This process continues again and again until the nerve signal goes all the way to the end of the fiber. In each case in which positive feedback is useful, the positive feedback itself is part of an overall negative feedback process. For example, in the case of blood clotting, the positive feedback clotting process is a negative feedback process for maintenance of normal blood volume. Also, the positive feedback that causes nerve signals allows the nerves to participate in thousands of negative feedback nervous control systems. More Complex Types of Control Systems— Adaptive Control Later in this text, when we study the nervous system, we shall see that this system contains great numbers of interconnected control mechanisms. Some are simple feedback systems similar to those already discussed. Many are not. For instance, some movements of the body occur so rapidly that there is not enough time for nerve signals to travel from the peripheral parts of the body all the way to the brain and then back to the periphery again to control the movement. Therefore, the brain uses a principle called feed-forward control to cause required muscle contractions. That is, sensory nerve signals from the moving parts apprise the brain whether the movement is performed correctly. If not, the brain corrects the feed-forward signals that it sends to the muscles the next time the movement is required. Then, if still further correction is needed, this will be done again for subsequent movements. This is called adaptive control. Adaptive control, in a sense, is delayed negative feedback. Thus, one can see how complex the feedback control systems of the body can be. A person’s life depends on all of them. Therefore, a major share of this text is devoted to discussing these life-giving mechanisms. Summary—Automaticity of the Body The purpose of this chapter has been to point out, first, the overall organization of the body and, second, the means by which the different parts of the body operate in harmony.To summarize, the body is actually a social order of about 100 trillion cells organized into different functional structures, some of which are called organs. Each functional structure contributes its share to the maintenance of homeostatic conditions in the extracellular fluid, which is called the internal environment. As long as normal conditions are maintained in this internal environment, the cells of the body continue to live and function properly. Each cell benefits from homeostasis, and in turn, each cell contributes its share toward the maintenance of homeostasis. This reciprocal interplay provides continuous automaticity of the body until one or more functional systems lose their ability to contribute their share of function.When this happens, all the cells of the body suffer. Extreme dysfunction leads to death; moderate dysfunction leads to sickness.
- Figure 49-3 Sympathetic and parasympathetic divisions of the autonomic nervous system. Sympathetic preganglionic neurons are clustered in ganglia in the sympathetic chain alongside the spinal cord extending from the first thoracic spinal segment to upper lumbar segments. Parasympathetic preganglionic neurons are located within the brain stem and in segments S2-S4 of the spinal cord. The major targets of autonomic control are shown here. The Autonomic Nervous System and the Hypothalamus Susan Iversen Leslie Iversen Clifford B. Saper WHEN WE ARE FRIGHTENED our heart races, our breathing becomes rapid and shallow, our mouth becomes dry, our muscles tense, our palms become sweaty, and we may want to run. These bodily changes are mediated by the autonomic nervous system , which controls heart muscle, smooth muscle, and exocrine glands. The autonomic nervous system is distinct from the somatic nervous system , which controls skeletal muscle. As we shall learn in the next chapter, even though the neural control of emotion involves several regions, including the amygdala and the limbic association areas of the cerebral cortex, they all work through the hypothalamus to control the autonomic nervous system. The hypothalamus coordinates behavioral response to insure bodily homeostasis , the constancy of the internal environment. The hypothalamus, in turn, acts on three major systems: the autonomic nervous system, the endocrine system, and an ill-defined neural system concerned with motivation. In this chapter we shall first examine the autonomic nervous system and then go on to consider the hypothalamus. In the next two chapters, we shall examine emotion and motivation, behavioral states that depend greatly on autonomic and hypothalamic mechanisms. The Autonomic Nervous System Is a Visceral and Largely Involuntary Sensory and Motor System In contrast to the somatic sensory and motor systems, which we considered in Parts IV and V of this book, the autonomic nervous system is a visceral sensory and motor system. Virtually all visceral reflexes are mediated by local circuits in the brain stem or spinal cord. Although these reflexes are regulated by a network of central autonomic control nuclei in the brain stem, hypothalamus, and forebrain, these visceral reflexes are not under voluntary control, nor do they impinge on consciousness, with few exceptions. The autonomic nervous system is thus also referred to as the involuntary motor system, in contrast to the voluntary (somatic) motor system. The autonomic nervous system has three major divisions: sympathetic, parasympathetic, and enteric. The sympathetic and parasympathetic divisions innervate cardiac muscle, smooth muscle, and glandular tissues and mediate a variety of visceral reflexes. These two divisions include the sensory neurons associated with spinal and cranial nerves, the preganglionic and postganglionic motor neurons, and the central nervous system circuitry that connects with and modulates the sensory and motor neurons. The enteric division has greater autonomy than the other two divisions and comprises a largely self-contained system, with only minimal connections to the rest of the nervous system. It consists of sensory and motor neurons in the gastrointestinal tract that mediate digestive reflexes. The American physiologist Walter B. Cannon first proposed that the sympathetic and parasympathetic divisions have distinctly different functions. He argued that the parasympathetic nervous system is responsible for rest and digest , maintaining basal heart rate, respiration, and metabolism under normal conditions. The sympathetic nervous system, on the other hand, governs the emergency reaction, or fight-or-flight reaction. In an emergency the body needs to respond to sudden changes in the external or internal environment, be it emotional stress, combat, athletic competition, severe change in temperature, or blood loss. For a person to respond effectively, the sympathetic nervous system increases output to the heart and other viscera, the peripheral vasculature and sweat glands, and the piloerector and certain ocular muscles. An animal whose sympathetic nervous system has been experimentally eliminated can only survive if sheltered, kept warm, and not exposed to stress or emotional stimuli. Such an animal cannot, however, carry out strenuous work or fend for itself; it cannot mobilize blood sugar from the liver quickly and does not react to cold with normal vasoconstriction or elevation of body heat. The relationship between the sympathetic and parasympathetic pathways is not as simple and as independent as suggested by Cannon, however. Both divisions are tonically active and operate in conjunction with each other and with the somatic motor system to regulate most behavior, be it normal or emergency. Although several visceral functions are controlled predominantly by one or the other division, and although both the sympathetic and parasympathetic divisions often exert opposing effects on innervated target tissues, it is the balance of activity between the two that helps maintain an internal stable environment in the face of changing external conditions. The idea of a stable internal environment in the face of changing external conditions was first proposed in the nineteenth century by the French physiologist Claude Bernard. This idea was developed further by Cannon, who put forward the concept of homeostasis as the complex physiological mechanisms that maintain the internal milieu. In his classic book The Wisdom of the Body published in 1932, Cannon introduced the concept of negative feedback regulation as a key homeostatic mechanism and outlined much of our current understanding of the functions of the autonomic nervous system. If a state remains steady, it does so because any change is automatically met by increased effectiveness of the factor or factors that resist the change. Consider, for example, thirst when the body lacks water; the discharge of adrenaline, which liberates sugar from the liver when the concentration of sugar in the blood falls below a critical point; and increased breathing, which reduces carbonic acid when the blood tends to shift toward acidity. Cannon further proposed that the autonomic nervous system, under the control of the hypothalamus, is an important part of this feedback regulation. The hypothalamus regulates many of the neural circuits that mediate the peripheral components of emotional states: changes in heart rate, blood pressure, temperature, and water and food intake. It also controls the pituitary gland and thereby regulates the endocrine system. The Visceral Motor System Overview The visceral (or autonomic) motor system controls involuntary functions mediated by the activity of smooth muscle fibers, cardiac muscle fibers, and glands. The system comprises two major divisions, the sympathetic and parasympathetic subsystems (the specialized innervation of the gut provides a further semi-independent component and is usually referred to as the enteric nervous system). Although these divisions are always active at some level, the sympatheticsystem mobilizes the body's resources for dealing with challenges of one sort or another. Conversely, parasympathetic system activity predominates during states of relative quiescence, so that energy sources previously expended can be restored. This continuous neural regulation of the expenditure and replenishment of the body's resources contributes importantly to the overall physiological balance of bodily functions called homeostasis. Whereas the major controlling centers for somatic motor activity are the primary and secondary motor cortices in the frontal lobes and a variety of related brainstem nuclei, the major locus of central control in the visceral motor system is the hypothalamus and the complex (and ill-defined) circuitry that it controls in the brainstem tegmentum and spinal cord. The status of both divisions of the visceral motor system is modulated by descending pathways from these centers to preganglionic neurons in the brainstem and spinal cord, which in turn determine the activity of the primary visceral motor neurons in autonomic ganglia. The autonomic regulation of several organ systems of particular importance in clinical practice (including cardiovascular function, control of the bladder, and the governance of the reproductive organs) is considered in more detail as specific examples of visceral motor control. Early Studies of the Visceral Motor System Although humans must always have been aware of involuntary motor reactions to stimuli in the environment (e.g., narrowing of the pupil in response to bright light, constriction of superficial blood vessels in response to cold or fear, increased heart rate in response to exertion), it was not until the late nineteenth century that the neural control of these and other visceral functions came to be understood in modern terms. The researchers who first rationalized the workings of the visceral motor system were Walter Gaskell and John Langley, two British physiologists at Cambridge University. Gaskell, whose work preceded that of Langley, established the overall anatomy of the system and carried out early physiological experiments that demonstrated some of its salient functional characteristics (e.g., that the heartbeat of an experimental animal is accelerated by stimulating the outflow of the upper thoracic spinal cord segments). Based on these and other observations, Gaskell concluded in 1866 that “every tissue is innervated by two sets of nerve fibers of opposite characters,” and he further surmised that these actions showed “the characteristic signs of opposite chemical processes.” Langley went on to establish the function of autonomic ganglia (which harbor the primary visceral motor neurons), defined the terms “preganglionic” and “postganglionic” (see next section), and coined the phrase autonomic nervous system (which is basically a synonym for “visceral motor system”; the terms are used interchangeably). Langley's work on the pharmacology of the autonomic system initiated the classical studies indicating the roles of acetylcholine and the catecholamines in autonomic function, and in neurotransmitter function more generally (see Chapter 6). In short, Langley's ingenious physiological and anatomical experiments established in detail the general proposition put forward by Gaskell on circumstantial grounds. The third major figure in the pioneering studies of the visceral motor system was Walter Cannon at Harvard Medical School, who during the early to mid-1900s devoted his career to understanding autonomic functions in relation to homeostatic mechanisms generally, and to the emotions and higher brain functions in particular (see Chapter 29). He also established the effects of denervation in the visceral motor system, laying some of the basis for much further work on what is now referred to as “neuronal plasticity” (see Chapter 25) Summary Sympathetic and parasympathetic ganglia, which contain the primary visceral-motor neurons that innervate smooth muscles, cardiac muscle, and glands, are controlled by preganglionic neurons in the spinal cord and brainstem. The sympathetic preganglionic neurons that govern ganglion cells in the sympatheticdivision of the visceral motor system arise from neurons in the thoracic and upper lumbar segments of the spinal cord; parasympathetic preganglionic neurons, in contrast, are located in the brainstem and sacral spinal cord. Sympathetic ganglion cells are distributed in the sympathetic chain (paravertebral) and prevertebral ganglia, whereas the parasympathetic motor neurons are more widely distributed in ganglia that lie within or near the organs they control. Most autonomic targets receive inputs from both the sympathetic and parasympathetic systems, which act in a generally antagonistic fashion. The diversity of autonomic functions is achieved primarily by different types of receptors for the two primary classes of postganglionic autonomic neurotransmitters, norepinephrine in the case of the sympathetic division and acetylcholine in the parasympathetic division. The visceral motor system is regulated by sensory feedback provided by dorsal root and cranial nerve sensory ganglion cells that make local reflex connections in the spinal cord or brainstem and project to the nucleus of the solitary tract in the brainstem, and by descending pathways from the hypothalamus and brainstem tegmentum, the major controlling centers of the visceral motor system (and of homeostasis more generally). The importance of the visceral motor control of organs such as the heart, bladder, and reproductive organs—and the many pharmacological means of modulating autonomic function—have made visceral motor control a central theme in clinical medicine.
- Figure 49-1 Anatomical organization of the somatic and autonomic motor pathways. A. In the somatic motor system, effector motor neurons in the central nervous system project directly to skeletal muscles. B. In the autonomic motor system, the effector motor neurons are located in ganglia outside the central nervous system and are controlled by preganglionic central neurons. The Motor Neurons of the Autonomic Nervous System Lie Outside the Central Nervous System In the somatic motor system the motor neurons are part of the central nervous system: They are located in the spinal cord and brain stem and project directly to skeletal muscle. In contrast, the motor neurons of the sympathetic and parasympathetic motor systems are located outside the spinal cord in the autonomic ganglia. The autonomic motor neurons (also known as postganglionic neurons ) are activated by the axons of central neurons (the preganglionic neurons ) whose cell bodies are located in the spinal cord or brain stem, much as are the somatic motor neurons. Thus, in the visceral motor system a synapse (in the autonomic ganglion) is interposed between the efferent neuron in the central nervous system and the peripheral target (Figure 49-1). The sympathetic and parasympathetic nervous systems have clearly defined sensory components that provide input to the central nervous system and play an important role in autonomic reflexes. In addition, some sensory fibers that project to the spinal cord also send a branch to autonomic ganglia, thus forming reflex circuits that control some visceral autonomic functions. The innervation of target tissues by autonomic nerves also differs markedly from that of skeletal muscle by somatic motor nerves. Unlike skeletal muscle, which has specialized postsynaptic regions (the end-plates; see Chapter 14), target cells of the autonomic nerve fibers have no specialized postsynaptic sites. Nor do the postganglionic nerve endings have presynaptic specializations such as the active zones of somatic motor neurons. Instead, the nerve endings have several swellings ( varicosities ) where vesicles containing transmitter substances accumulate (see Chapter 15). Synaptic transmission therefore occurs at multiple sites along the highly branched axon terminals of autonomic nerves. The neurotransmitter may diffuse for distances as great as several hundred nanometers to reach its targets. In contrast to the point-to-point contacts made in the somatic motor system, neurons in the autonomic motor system exert a more diffuse control over target tissues, so that a relatively small number of highly branched motor fibers can regulate the function of large masses of smooth muscle or glandular tissue.
- course of preganglionic and postganglionic sympathetic fibers innervating different organs. (A) Organs in the head. (B) Organs in the chest. (C) Organs in the abdomen. (D) Adrenal gland. Also note that, at each level, the axons of the postganglionic neurons in the paravertebral ganglia re-enter the corresponding spinal nerves through gray rami, travel within or along the spinal nerve, and innervate the blood vessels, sweat glands, and erectile muscle of hair follicles. Figure 21.2. Organization of the preganglionic spinal outflow to sympathetic ganglia. (A) General organization of the sympathetic division of the visceral motor system in the spinal cord and the preganglionic outflow to the sympathetic ganglia that contain the primary visceral motor neurons. (B) Cross section of thoracic spinal cord at the level indicated, showing location of the sympathetic preganglionic neurons in the intermediolateral cell column of the lateral horn. Sympathetic Pathways Convey Thoracolumbar Outputs to Ganglia Alongside the Spinal Cord Preganglionic sympathetic neurons form a column in the intermediolateral horn of the spinal cord extending from the first thoracic spinal segment to rostral lumbar segments. The axons of these neurons leave the spinal cord in the ventral root and initially run together in the spinal nerve. They then separate from the somatic motor axons and project (in small bundles called white myelinated rami ) to the ganglia of the sympathetic chains , which lie along each side of the spinal cord (Figure 49-2). Axons of preganglionic neurons exit the spinal cord at the level at which their cell bodies are located, but they may innervate sympathetic ganglia situated either more rostrally or more caudally by traveling in the sympathetic nerve trunk that connects the ganglia (Figure 49-2). Most of the preganglionic axons are relatively slow-conducting, small-diameter myelinated fibers. Each preganglionic fiber forms synapses with many postganglionic neurons in different ganglia. Overall, the ratio of preganglionic fibers to postganglionic fibers in the sympathetic nervous system is about 1:10. This divergence permits coordinated activity in sympathetic neurons at several different spinal levels. The axons of postganglionic neurons are largely unmyelinated and exit the ganglia in the gray unmyelinated rami. The postganglionic cells that innervate structures in the head are located in the superior cervical ganglion, which is a rostral extension of the sympathetic chain. The axons of these cells travel along branches of the carotid arteries to their targets in the head. The postganglionic fibers innervating the rest of the body travel in spinal nerves to their targets; in an average spinal nerve about 8% of the fibers are sympathetic postganglionic axons. Some neurons of the cervical and upper thoracic ganglia innervate cranial blood vessels, sweat glands, and hair follicles; others innervate the glands and visceral organs of the head and chest, including the lacrimal and salivary glands, heart, lungs, and blood vessels. Neurons in the lower thoracic and lumbar paravertebral ganglia innervate peripheral blood vessels, sweat glands, and pilomotor smooth muscle (Figure 49-3). Some preganglionic fibers pass through the sympathetic ganglia and branches of the splanchnic nerves to synapse on neurons of the prevertebral ganglia , which include the coeliac ganglion and the superior and inferior mesenteric ganglia (Figure 49-3). Neurons in these ganglia innervate the gastrointestinal system and the accessory gastrointestinal organs, including the pancreas and liver, and also provide sympathetic innervation of the kidneys, bladder, and genitalia. Another group of preganglionic axons runs in the thoracic splanchnic nerve into the abdomen and innervates the adrenal medulla, which is an endocrine gland, secreting both epinephrine and norepinephrine into circulation. The cells of the adrenal medulla are developmentally and functionally related to postganglionic sympathetic neurons.
- Figure 21.3. Organization of the preganglionic outflow to parasympathetic ganglia. (A) Dorsal view of brainstem showing the location of the nuclei of the cranial part of the parasympathetic division of the visceral motor system. (B) Cross section of the brainstem at the relevant levels [indicated by blue lines in (A)] showing location of these parasympathetic nuclei. (C) Main features of the parasympathetic preganglionics in the sacral segments of the spinal cord. (D) Cross section of the sacral spinal cord showing location of sacral preganglionic neurons Parasympathetic Pathways Convey Outputs From the Brain Stem Nuclei and Sacral Spinal Cord to Widely Dispersed Ganglia The central, preganglionic cells of the parasympathetic nervous system are located in several brain stem nuclei and in segments S2-S4 of the sacral spinal cord (Figure 49-3). The axons of these cells are quite long because parasympathetic ganglia lie close to or are actually embedded in visceral target organs. In contrast, sympathetic ganglia are located at some distance from their targets. The preganglionic parasympathetic nuclei in the brain stem include the Edinger-Westphal nucleus (associated with cranial nerve III), the superior and inferior salivary nuclei (associated with cranial nerves VII and IX, respectively), and the dorsal vagal nucleus and the nucleus ambiguus (both associated with cranial nerve X). Preganglionic axons exiting the brain stem through cranial nerves III, VII, and IX and project to postganglionic neurons in the ciliary, pterygopalatine, submandibular, and otic ganglia (Figure 49-3). Parasympathetic preganglionic fibers from the dorsal vagal nucleus project via nerve X to postganglionic neurons embedded in thoracic and abdominal targets—the stomach, liver, gall bladder, pancreas, and upper intestinal tract (Figure 49-3). Neurons of the ventrolateral nucleus ambiguus provide the principal parasympathetic innervation of the cardiac ganglia, which innervate the heart, esophagus, and respiratory airways. In the sacral spinal cord the parasympathetic preganglionic neurons occupy the intermediolateral column. Axons of spinal parasympathetic neurons leave the spinal cord through the ventral roots and project in the pelvic nerve to the pelvic ganglion plexus. Pelvic ganglion neurons innervate the descending colon, bladder, and external genitalia (Figure 49-3). The sympathetic nervous system innervates tissues throughout the body, but the parasympathetic distribution is more restricted. There is also less divergence, with an average ratio of preganglionic to postganglionic fibers of about 1:3; in some tissues the numbers may be nearly equal.
- Figure 21.5. Organization of sensory input to the visceral motor system. (A) Afferent input from the cranial nerves relevant to visceral sensation (as well as afferent input ascending from the spinal cord not shown here) converge on the nucleus of the solitary tract. (B) Cross section of the brainstem showing the location of the nucleus of the solitary tract, which is so-named because of its association with the tract of the myelinated axons that supply it. Sensory Components of the Visceral Motor System The visceral motor system clearly requires sensory feedback to control and modulate its many functions. As in the case of somatic sensory modalities (see Chapters 9 and 10 ), the cell bodies of the visceral afferent fibers lie in the dorsal root ganglia or the sensory ganglia associated with cranial nerves (in this case, the vagus, glossopharyngeal, and facial nerves) ( Figure 21.5A ). The neurons in the dorsal root ganglia send an axon peripherally to end in sensoryreceptor specializations, and an axon centrally to terminate in a part of the dorsal horn of the spinal cord near the lateral horn, where the preganglionic neurons of both sympathetic and parasympathetic divisions are located. In addition to making local reflex connections, branches of these visceral sensoryneurons also travel rostrally to innervate nerve cells in the brainstem; in this case, however, the target is the nucleus of the solitary tract in the upper medulla ( Figure 21.5B ). The afferents from viscera in the head and neck that enter the brainstem via the cranial nerves also terminate in the nucleus of the solitary tract (see Figure 21.5B ). This nucleus, as described in the next section, integrates a wide range of visceral sensory information and transmits to the hypothalamus and to the relevant motor nuclei in the brainstem tegmentum. Sensory fibers related to the viscera convey only limited information to consciousness—primarily pain. Nonetheless, the visceral afferent information of which we are not aware is essential for the functioning of autonomic reflexes. Specific examples described in more detail later in the chapter include afferent information relevant to cardiovascular control, to the control of the bladder, and to the governance of sexual functions (although sexual reflexes are, exceptionally, not mediated by the nucleus of the solitary tract) Sensory Inputs Produce a Wide Range of Visceral Reflexes To maintain homeostasis the autonomic nervous system responds to many different types of sensory inputs. Some of these are somatosensory. For example, a noxious stimulus activates sympathetic neurons that regulate local vasoconstriction (necessary to reduce bleeding when the skin is broken). At the same time, the stimulus activates nociceptive afferents in the spinothalamic tract with axon collaterals to an area in the rostral ventrolateral medulla that coordinates reflexes. These inputs cause widespread sympathetic activation that increases blood pressure and heart rate to protect arterial perfusion pressure and prepares the individual for vigorous defense. Homeostasis also requires important information about the internal state of the body. Much of this information from the thoracic and abdominal cavities reaches the brain via the vagus nerve. The glossopharyngeal nerve also conveys visceral sensory information from the head and neck. Both of these nerves and the facial nerve relay special visceral sensory information about taste (a visceral chemosensory function) from the oral cavity. All of these visceral sensory afferents synapse in a topographic fashion in the nucleus of the solitary tract. Taste information is represented most anteriorly; gastrointestinal information, in an intermediate position; cardiovascular inputs, caudomedially; and respiratory inputs, in the caudolateral part of the nucleus.
- Neurotransmission in the Visceral Motor System The neurotransmitter functions of the visceral motor system are of enormous importance in clinical practice, and drugs that act on the autonomic system are among the most important in the clinical armamentarium. Moreover, autonomic transmitters have played a major role in the history of efforts to understand synaptic function. Consequently, neurotransmission in the visceral motor system deserves special comment (see also Chapter 6 ). Acetylcholine is the primary neurotransmitter of both sympathetic and parasympathetic preganglionic neurons. Nicotinic receptors on autonomic ganglion cells are ligand-gated ion channels that mediate a so-called fast EPSP (much like nicotinic receptors at the neuromuscular junction). In contrast, muscarinic acetylcholine receptors on ganglion cells are members of the 7-transmembrane G protein-linked receptor family, and they mediate slower synaptic responses (see Chapters 7 and 8 ). The primary action of muscarinic receptors in autonomic ganglion cells is to close K+ channels, making the neurons more excitable and generating a prolonged EPSP. As a result of these two acetylcholine receptor types, ganglionic synapses mediate both rapid excitation and a slower modulation of autonomic ganglion cell activity. The postganglionic effects of autonomic ganglion cells on their smooth muscle, cardiac muscle, or glandular targets are mediated by two primary neurotransmitters: norepinephrine (NE) and acetylcholine (ACh). For the most part, sympathetic ganglion cells release norepinephrine onto their targets (a notable exception is the cholinergic sympathetic innervation of sweat glands), whereas parasympathetic ganglion cells typically release acetylcholine. As expected from the foregoing account, these two neurotransmitters usually have opposing effects on their target tissue—contraction versus relaxation of smooth muscle, for example. As described in Chapters 6 to 8 , the specific effects of either ACh or NE are determined by the type of receptor expressed in the target tissue, and the downstream signaling pathways to which these receptors are linked. Peripheral sympathetic targets generally have two subclasses of noradrenergic receptors in their cell membranes, referred to as α and β receptors. Like muscarinic ACh receptors, both α and β receptors and their subtypes belong to the 7-transmembrane G-protein-coupled class of cell surface receptors. The different distribution of these receptors in sympathetic targets allows for a variety of postsynaptic effects mediated by norepinephrine released from postganglionic sympathetic nerve endings ( Table 21.2 ). The effects of acetylcholine released by parasympathetic ganglion cells onto smooth muscles, cardiac muscle, and glandular cells also vary according to the subtypes of muscarinic cholinergic receptors found in the peripheral target ( Table 21.3 ). The two major subtypes are known as M1 and M2 receptors, M1 receptors being found primarily in the gut and M2 receptors in the cardiovascular system (another subclass of muscarinic receptors, M3, occurs in both smooth muscle and glandular tissues). Muscarinic receptors are coupled to a variety of intracellular signal transduction mechanisms that modify K+ and Ca2+channel conductances. They can also activate nitric oxide synthase, which promotes the local release of NO in some parasympathetic target tissues (see, for example, the section on autonomic control of sexual function). In contrast to the relatively restricted responses generated by norepinephrine and acetylcholine released by sympathetic and parasympathetic ganglion cells, respectively, neurons of the enteric nervous system achieve an enormous diversity of target effects by virtue of many different neurotransmitters, most of which are neuropeptides associated with specific cell groups in either the myenteric or submucous plexuses mentioned earlier. The details of these agents and their actions are beyond the scope of this introductory account Box 49-1 First Isolation of a Chemical Transmitter The existence of chemical messengers was first postulated by John Langley and Henry Dale and their students on the basis of their pharmacological studies dating from the beginning of the century. However, convincing evidence for a neurotransmitter was not provided until 1920, when Otto Loewi, in a simple but decisive experiment, examined the autonomic innervation of two isolated, beating frog hearts. In his own words: The night before Easter Sunday of that year I awoke, turned on the light, and jotted down a few notes on a tiny slip of paper. Then I fell asleep again. It occurred to me at six o'clock in the morning that during the night I had written down something most important, but I was unable to decipher the scrawl. The next night, at three o'clock, the idea returned. It was the design of an experiment to determine whether or not the hypothesis of chemical transmission that I had uttered seventeen years ago was correct. I got up immediately, went to the laboratory, and performed a simple experiment on a frog heart according to the nocturnal design. I have to describe briefly this experiment since its results became the foundation of the theory of chemical transmission of the nervous impulse. The hearts of two frogs were isolated, the first with its nerves, the second without. Both hearts were attached to Straub cannulas filled with a little Ringer solution. The vagus nerve of the first heart was stimulated for a few minutes. Then the Ringer solution that had been in the first heart during the stimulation of the vagus was transferred to the second heart. It slowed and its beat diminished just as if its vagus had been stimulated. Similarly, when the accelerator nerve was stimulated and the Ringer from this period transferred, the second heart speeded up and its beat increased. These results unequivocally proved that the nerves do not influence the heart directly but liberate from their terminals specific chemical substances which, in their turn, cause the well-known modifications of the function of the heart characteristic of the stimulation of its nerves. Loewi called this substance Vagusstoff (vagus substance). Soon after, Vagusstoff was identified chemically as acetylcholine. The nucleus of the solitary tract distributes visceral sensory information within the brain along three main pathways. Some neurons in the nucleus of the solitary tract directly innervate preganglionic neurons in the medulla and spinal cord, triggering direct autonomic reflexes. For example, there are direct inputs from the nucleus of the solitary tract to vagal motor neurons controlling esophageal and gastric motility, which are important for ingesting food. Also, projections from the nucleus of the solitary tract to the spinal cord are involved in respiratory reflex responses to lung inflation. Other neurons in the nucleus project to the lateral medullary reticular formation, where they engage populations of premotor neurons that organize more complex, patterned autonomic reflexes. For example, groups of neurons in the rostral ventrolateral medulla control blood pressure by regulating both blood flow to different vascular beds and vagal tone in the heart to modulate heart rate. Other groups of neurons control complex responses such as vomiting and respiratory rhythm (a somatic motor response that has an important autonomic component and that depends critically on visceral sensory information). The third main projection from the nucleus of the solitary tract provides visceral sensory input to a network of cell groups that extend from the pons and midbrain up through the hypothalamus, amygdala, and cerebral cortex. This network coordinates autonomic responses and integrates them into ongoing patterns of behavior. These will be described in more detail after we consider more elementary autonomic reflexes. Autonomic Neurons Use a Variety of Chemical Transmitters Autonomic ganglion cells receive and integrate inputs from both the central nervous system (through preganglionic nerve terminals) and the periphery (through branches of sensory nerves that terminate in the ganglia). Most of the sensory fibers are nonmyelinated and may release neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP), onto ganglion cells. Preganglionic fibers primarily use ACh and norepinephrine as transmitters. Ganglionic Transmission Involves Both Fast and Slow Synaptic Potentials Preganglionic activity induces both brief and prolonged responses from postganglionic neurons. ACh released from preganglionic terminals evokes fast excitatory postsynaptic potentials (EPSPs) mediated by nicotinic ACh receptors. The fast EPSP is often large enough to generate an action potential in the postganglionic neuron, and it is thus regarded as the principal synaptic pathway for ganglionic transmission in both the sympathetic and parasympathetic systems. ACh also evokes slow EPSPs and inhibitory postsynaptic potentials (IPSPs) in postganglionic neurons. These slow potentials can modulate the excitability of these cells. They have been most often studied in sympathetic ganglia but are also known to occur in some parasympathetic ganglia. Slow EPSPs or IPSPs are mediated by muscarinic ACh receptors (Figure 49-6). The slow excitatory potential results when Na+ and Ca2+ channels open and M-type K+ channels close. The M-type channels are normally active at the resting membrane potential, so their closure leads to membrane depolarization (Chapter 13). The slow inhibitory potential results from the opening of K+ channels, allowing K+ ions to flow out of the nerve terminals, resulting in hyperpolarization. The fast cholinergic EPSP reaches a maximum within 10-20 ms; the slow cholinergic synaptic potentials take up to half a second to reach their maximum and last for a second or more (Figure 49-6). Even slower synaptic potentials, lasting up to a minute, are evoked by neuropeptides, a variety of which are present in the terminals of preganglionic neurons and sensory nerve endings. The actions of one peptide have been studied in detail and reveal important features of peptidergic transmission. In some, but not all, preganglionic nerve terminals in bullfrog sympathetic ganglia, ACh is colocalized with a luteinizing hormone-releasing hormone (LHRH)-like peptide. High-frequency stimulation of the preganglionic nerves causes the peptide to be released, evoking a slow, long-lasting EPSP in all postganglionic neurons (Figure 49-6), even those not directly innervated by the peptidergic fibers. The peptide must diffuse over considerable distances to influence distant receptive neurons. The slow peptidergic EPSP, like the slow cholinergic excitatory potential, also results from the closure of M-type channels and the opening of Na+ and Ca2+ channels. The peptidergic excitatory potential alters the excitability of autonomic ganglion cells for long periods after intense activation of preganglionic inputs. No mammalian equivalent of the actions of the LHRH-like peptide in amphibians has yet been identified, but the neuropeptide substance P released from sensory afferent terminals in mammals evokes a similar slow, long-lasting EPSP. Norepinephrine and Acetylcholine Are the Predominant Transmitters in the Autonomic Nervous System Most postganglionic sympathetic neurons release norepinephrine, which acts on a variety of different adrenergic receptors. There are five major types of adrenergic receptors, and these are the target for several medically important drugs (Table 49-1). ATP and Adenosine Have Potent Extracellular Actions Adenosine triphosphate (ATP) is an important cotransmitter with norepinephrine in many postganglionic sympathetic neurons. By acting on ATP-gated ion channels (P2 purinergic receptors), it is responsible for some of the fast responses seen in target tissues (Table 49-1). The proportion of ATP to norepinephrine varies considerably in different sympathetic nerves. The ATP component is relatively minor in nerves to blood vessels in the rat tail and rabbit ear, while the responses of guinea pig submucosal arterioles to sympathetic stimulation appear to be mediated solely by ATP. The nucleotide adenosine is formed from the hydrolysis of ATP and is recognized by P1 purinergic receptors (Table 49-1) located both pre- and postjunctionally. It is thought to play a modulatory role in autonomic transmission, particularly in the sympathetic system. Adenosine may dampen sympathetic function after intense sympathetic activation by activating receptors on sympathetic nerve endings that inhibit further norepinephrine and ATP release. Adenosine also has inhibitory actions in cardiac and smooth muscle that tend to oppose the excitatory actions of norepinephrine. Many Different Neuropeptides Are Present in Autonomic Neurons Neuropeptides are colocalized with norepinephrine and ACh in autonomic neurons. Cholinergic preganglionic neurons in the spinal cord and brain stem and their terminals in autonomic ganglia may contain enkephalins, neurotensin, somatostatin, or substance P. Noradrenergic postganglionic sympathetic neurons may also express a variety of neuropeptides. Neuropeptide Y is present in as many as 90% of the cells and modulates sympathetic transmission. In tissues in which the nerve endings are distant from their targets (more than 60 nm, as for the rabbit ear artery), neuropeptide Y potentiates both the purinergic and adrenergic components of the tissue response, probably by acting postsynaptically. In contrast, in tissues with dense sympathetic innervation and where the target is closer (20 nm, such as the vas deferens), neuropeptide Y acts presynaptically to inhibit release of ATP and norepinephrine, thus dampening the tissue response. The peptides galanin and dynorphin are often found with neuropeptide Y in sympathetic neurons, which can contain several neuropeptides. Cholinergic postganglionic sympathetic neurons commonly contain CGRP and vasoactive intestinal polypeptide (VIP) (Figure 49-7).
- Visceral Motor Reflex Functions Many examples of specific autonomic functions could be used to illustrate in more detail how the visceral motor system operates. The three outlined here—control of cardiovascular function, control of the bladder, and control of sexual function—have been chosen primarily because of their importance in human physiology and clinical practice Autonomic Regulation of Cardiovascular Function The cardiovascular system is subject to precise reflex regulation so that an appropriate supply of oxygenated blood can be reliably provided to different body tissues under a wide range of circumstances. The sensory monitoring for this critical homeostatic process entails primarily mechanical (barosensory) information about pressure in the arterial system and, secondarily, chemical (chemosensory) information about the level of oxygen and carbon dioxide in the blood. The parasympathetic and sympathetic activity relevant to cardiovascular control is determined by the information supplied by these sensors. The mechanoreceptors (called baroreceptors) are located in the heart and major blood vessels; the chemoreceptors are located primarily in the carotid bodies, which are small, highly specialized organs located at the bifurcation of the common carotid arteries (some chemosensory tissue is also found in the aorta). The nerve endings in baroreceptors are activated by deformation as the elastic elements of the vessel walls expand and contract. The chemoreceptors in the carotid bodies and aorta respond directly to the partial pressure of oxygen and carbon dioxide in the blood. Both afferent systems convey their status via the vagus nerve to the nucleus of the solitary tract ( Figure 21.7 ), which relays this information to the hypothalamus and the relevant brainstem tegmental nuclei (see earlier). The afferent information from changes in arterial pressure and blood gas levels reflexively modulates the activity of the relevant visceral motor pathways and, ultimately, of target smooth and cardiac muscles and other more specialized structures. For example, a rise in blood pressure activates baroreceptors that, via the pathway illustrated in Figure 21.7 , inhibit the tonic activity of sympathetic preganglionic neurons in the spinal cord. In parallel, the pressure increase stimulates the activity of the parasympathetic preganglionic neurons in the dorsal motor nucleus of the vagus and the nucleus ambiguus that influence heart rate. The carotid chemoreceptors also have some influence, but this is a less important drive than that stemming from the baroreceptors. As a result of this shift in the balance of sympathetic and parasympathetic activity, the stimulatory noradrenergic effects of postganglionic sympathetic innervation on the cardiac pacemaker and cardiac musculature is reduced (an effect abetted by the decreased output of catecholamines from the adrenal medulla and the decreased vasoconstrictive effects of sympathetic innervation on the peripheral blood vessels). At the same time, activation of the cholinergic parasympathetic innervation of the heart decreases the discharge rate of the cardiac pacemaker in the sinoatrial node and slows the ventricular conduction system. These parasympathetic influences are mediated by an extensive series of parasympathetic ganglia in and near the heart, which release acetylcholine onto cardiac pacemaker cells and cardiac muscle fibers. As a result of this combination of sympathetic and parasympathetic effects, heart rate and the effectiveness of the atrial and ventricular mycoardial contraction are reduced and the peripheral arterioles dilate, thus lowering the blood pressure. In contrast to this sequence of events, a drop in blood pressure, as might occur from blood loss, has the opposite effect, inhibiting parasympathetic activity while increasing sympathetic activity. As a result, norepinephrine is released from sympathetic postganglionic terminals, increasing the rate of cardiac pacemaker activity and enhancing cardiac contractility, at the same time increasing release of catecholamines from the adrenal medulla (which further augments these and many other sympathetic effects that enhance the response to this threatening situation). Norepinephrine released from the terminals of sympathetic ganglion cells also acts on the smooth muscles of the arterioles to increase the tone of the peripheral vessels, particularly those in the skin, subcutaneous tissues, and muscles, thus shunting blood away from these tissues to those organs where oxygen and metabolites are urgently needed to maintain function (e.g., brain, heart, and kidneys in the case of blood loss). If these reflex sympathetic responses fail to raise the blood pressure sufficiently (in which case the patient is said to be in shock), the vital functions of these organs begin to fail, often catastrophically. A more mundane circumstance that requires a reflex autonomic response to a fall in blood pressure is standing up. Rising quickly from a prone position produces a shift of some 300–800 milliliters of blood from the thorax and abdomen to the legs, resulting in a sharp (approximately 40%) decrease in the output of the heart. The adjustment to this normally occurring drop in blood pressure (called orthostatic hypotension) must be rapid and effective, as evidenced by the dizziness sometimes experienced in this situation. Indeed, normal individuals can briefly lose consciousness as a result of blood pooling in the lower extremities, which is the usual cause of fainting among healthy individuals who must stand still for abnormally long periods (the “Beefeaters” who guard Buckingham Palace, for example). The sympathetic innervation of the heart arises from the preganglionic neurons in the intermediolateral column of the spinal cord, extending from roughly the first through fifth thoracic segments (see Table 21.1 ). The primary visceral motor neurons are in the adjacent thoracic paravertebral and prevertebral ganglia of the cardiac plexus. The parasympathetic preganglionics, as already mentioned, are in the dorsal motor nucleus of the vagus nerve and the nucleus ambiguus, projecting to parasympathetic ganglia in and around the heart and great vessels
- Figure 21.4. Organization of the enteric component of the visceral motor system. (A) Sympathetic and parasympathetic innervation of the enteric nervous system, and the intrinsic neurons of the gut. (B) Detailed organization of nerve cell plexuses in the gut wall. The neurons of the submucus plexus (Meissner's plexus) are concerned with the secretory aspects of gut function, and the myenteric plexus (Auerbach's plexus) with the motor aspects of gut function (e.g., peristalsis). The Enteric Nervous System An enormous number of neurons are specifically associated with the gastrointestinal tract to control its many functions; indeed, more neurons are said to reside in the human gut than in the entire spinal cord. As already noted, the activity of the gut is modulated by both the sympathetic and the parasympathetic divisions of the visceral motor system. However, the gut also has an extensive system of nerve cells in its wall (as do its accessory organs such as the pancreas and gallbladder) that do not fit neatly into the sympathetic or parasympathetic divisions of the visceral motor system ( Figure 21.4A ). To a surprising degree, these neurons and the complex enteric plexuses in which they are found ( plexus means “network”) operate more or less independently according to their own reflex rules; as a result, many gut functions continue perfectly well without sympathetic or parasympathetic supervision (peristalsis, for example, occurs in isolated gut segments in vitro). Thus, most investigators prefer to classify the enteric nervous system as a separate component of the visceral motor system. The neurons in the gut wall include local and centrally projecting sensory neurons that monitor mechanical and chemical conditions in the gut, local circuit neurons that integrate this information, and motor neurons that influence the activity of the smooth muscles in the wall of the gut and glandular secretions (e.g., of digestive enzymes, mucus, stomach acid, and bile). This complex arrangement of nerve cells intrinsic to the gut is organized into: (1) the myenteric (or Auerbach's) plexus, which is specifically concerned with regulating the musculature of the gut; and (2) the submucus (or Meissner's) plexus, which is located, as the name implies, just beneath the mucus membranes of the gut and is concerned with chemical monitoring and glandular secretion ( Figure 21.4B ). As already mentioned, the preganglionic parasympathetic neurons that influence the gut are primarily in the dorsal motor nucleus of vagus nerve in the brainstem and the intermediate gray zone in the sacral spinal cord segments. The preganglionic sympathetic innervation that modulates the action of the gut plexuses derives from the thoraco-lumbar cord, primarily by way of the celiac, superior, and inferior mesenteric ganglia.
- Autonomic Regulation of the Bladder The autonomic regulation of the bladder provides a good example of the interplay between the voluntary motor system (obviously, we have voluntary control over urination), and the sympathetic and parasympathetic divisions of the visceral motor system, which operate involuntarily. The arrangement of afferent and efferent innervation of the bladder is shown in Figure 21.8 . The parasympathetic control of the bladder musculature, the contraction of which causes bladder emptying, originates with neurons in the sacral spinal cord segments (S2–S4) that innervate visceral motor neurons in parasympathetic ganglia in or near the bladder wall. Mechanoreceptors in the bladder wall supply visceral afferent information to the spinal cord and to higher autonomic centers in the brainstem (primarily the nucleus of the solitary tract), which in turn project to the various central coordinating centers for bladder function in the brainstem tegmentum and elsewhere. The sympathetic innervation of the bladder originates in the lower thoracic and upper lumbar spinal cord segments (T10-L2), the preganglionic axons running to sympathetic neurons in the inferior mesenteric ganglion and the ganglia of the pelvic plexus. The postganglionic fibers from these ganglia travel in the hypogastric and pelvic nerves to the bladder, where sympathetic activity causes the internal urethral sphincter to close (postganglionic sympathetic fibers also innervate the blood vessels of the bladder, and in males the smooth muscle fibers of the prostate gland). Stimulation of this pathway in response to a modest increase in bladder pressure from the accumulation of urine thus closes the internal sphincter and inhibits the contraction of the bladder wall musculature, allowing the bladder to fill. At the same time, moderate distension of the bladder inhibits parasympathetic activity (which would otherwise contract the bladder and allow the internal sphincter to open). When the bladder is full, afferent activity conveying this information centrally increases parasympathetic tone and decreases sympathetic activity, causing the internal sphincter muscle to relax and the bladder to contract. In this circumstance, the urine is held in check by the voluntary (somatic) motor innervation of the external urethral sphincter muscle (see Figure 21.8 ). The voluntary control of the external sphincter is mediated by α-motor neurons of the ventral horn in the sacral spinal cord segments (S2–S4), which cause the striated muscle fibers of the sphincter to contract. During bladder filling (and subsequently, until circumstances permit urination) these neurons are active, keeping the external sphincter closed and preventing bladder emptying. During urination (or “voiding,” as clinicians often call this process), this tonic activity is temporarily inhibited, leading to relaxation in the external sphincter muscle. Thus, urination results from the coordinated activity of sacral parasympathetic neurons and temporary inactivity of the α-motor neurons of the voluntary motor system. The central governance of these events stems from the rostral pons, the relevant pontine circuitry being referred to as the micturition center ( micturition is also “medicalese” for urination). This phrase implies more knowledge about the central control of bladder function than is actually available. As many as five other central regions have been implicated in the coordination of urinary functions, including the locus coeruleus, the hypothalamus, the septal nuclei, and several cortical regions. The cortical regions primarily concerned with the voluntary control of bladder function include the paracentral lobule, the cingulate gyrus, and the frontal lobes. This functional distribution accords the motor representation of perineal musculature in the medial part of the primary motorcortex (see Chapter 17 ), and the planning functions of the frontal lobes (see Chapter 26 ), which are equally pertinent to bodily functions (remembering to stop by the bathroom before going on a long trip, for instance). Importantly, paraplegic patients, or patients who have otherwise lost descending control of the sacral spinal cord, continue to exhibit autonomic regulation of bladder function, since urination is eventually stimulated reflexively at the level of the sacral cord by sufficient bladder distension. Unfortunately, this reflex is not efficient in the absence of descending motor control, resulting in a variety of problems in paraplegics and others with diminished or absent central control of bladder function. The major difficulty is incomplete bladder emptying, which often leads to chronic urinary tract infections from the culture medium provided by retained urine, and thus the need for an indwelling catheter to ensure adequate drainage Urogenital Reflexes The control of bladder emptying is unusual because it involves both involuntary autonomic reflexes and some voluntary control. The excitatory input to the bladder wall that causes contraction and promotes emptying is parasympathetic. Activation of parasympathetic postganglionic neurons in the pelvic ganglion plexus near to and within the bladder wall contracts the bladder's smooth muscle. These neurons are quiet when the bladder begins to fill but are activated reflexly by visceral afferents when the bladder is distended. The sympathetic nervous system relaxes the bladder smooth muscle. Axons of preganglionic sympathetic neurons project from the thoracic and upper lumbar spinal cord to the inferior mesenteric ganglion. From there, postganglionic fibers travel to the bladder in the hypogastric nerve. When the sympathetic system is activated by low-frequency firing in sensory afferents that respond to tension in the bladder wall, the parasympathetic neurons in the pelvic ganglion are inhibited, relaxing bladder smooth muscle and exciting the internal sphincter muscle. Thus, during bladder filling the sympathetic system promotes relaxation of the bladder wall directly while maintaining closure of the internal sphincter. Somatic motor neurons in the ventral horn of the sacral spinal cord innervate striated muscle fibers in the external urethral sphincter, causing it to contract. These motor neurons are stimulated by visceral afferents that are activated when the bladder is partially full. As the bladder fills, spinal sensory afferents relay this information to a region in the pons that coordinates micturition. This pontine area, sometimes called Barrington's nucleus after the British neurophysiologist who first described it, also receives important descending inputs from the forebrain concerning behavioral cues for emptying the bladder. Descending pathways from Barrington's nucleus cause coordinated inhibition of sympathetic and somatic systems, relaxing both sphincters. The onset of urinary flow through the urethra causes reflex contraction of the bladder that is under parasympathetic control. In patients with spinal cord injuries at the cervical or thoracic levels, the spinal reflex control of micturition remains intact, but the connections with the pons are severed. As a result, micturition cannot be voluntarily controlled. When it does occur as a spinal reflex resulting from bladder overfilling, urination is incomplete. As a result, urinary tract infections are common, and it may be necessary to empty the bladder mechanically by catheterization Sexual reflexes are organized in a pattern that is analogous to those controlling bladder function. Erectile tissue is controlled largely by the parasympathetic nervous system, involving neurons that produce nitrous oxide as their main mediator. Glandular secretion is also parasympathetically mediated. Ejaculation in males is caused by sympathetic control of the seminal vesicles and vas deferens, and emission involves control of striated muscles in the pelvic floor as well. Supraspinal inputs play an important role in producing the coordinated pattern of sexual response, although some simple sexual reflexes can be activated even after spinal transection (eg, penile erection can be elicited by local sensory stimuli).
- Autonomic Regulation of Sexual Function Much like control of the bladder, sexual responses are mediated by the coordinated activity of sympathetic, parasympathetic, and somatic innervation. Although these reflexes differ in detail in males and females, basic similarities allow the two sexes to be considered together, not only in humans but in mammals generally (see Chapter 30 ). The relevant autonomic effects include: (1) the mediation of vascular dilation, which causes penile or clitoral erection; (2) stimulation of prostatic or vaginal secretions; (3) smooth muscle contraction of the vas deferens during ejaculation or rhythmic vaginal contractions during orgasm in females; and (4) contractions of the somatic pelvic muscles that accompany orgasm in both sexes. Like the urinary tract, the reproductive organs receive preganglionic parasympathetic innervation from the sacral spinal cord, preganglionic sympathetic innervation from the outflow of the lower thoracic and upper lumbar spinal cord segments, and somatic motor innervation from α-motor neurons in the ventral horn of the lower spinal cord segments ( Figure 21.9 ). The sacral parasympathetic pathway controlling the sexual organs in both males and females originates in the sacral segments S2–S4 and reaches the target organs via the pelvic nerves. Activity of the postganglionic neurons in the relevant parasympathetic ganglia causes dilation of penile or clitoral arteries, and a corresponding relaxation of the smooth muscles of the venous (cavernous) sinusoids, which leads to expansion of the sinusoidal spaces. As a result, the amount of blood in the tissue is increased, leading to a sharp rise in the pressure and an expansion of the cavernous spaces (i.e., erection). The mediator of the smooth muscle relaxation leading to erection is not acetylcholine (as in most postganglionic parasympathetic actions), but nitric oxide (see Chapter 8 ). The drug sildenafil (Viagra®), for instance, acts by stimulating the activity of guanylate cyclase, which increases the conversion of GTP to cyclic GMP, mimicking the action of NO on the c-GMP pathway, thus enhancing the relaxation of the venous sinusoids and promoting erection in males with erectile dysfunction. Parasympathetic activity also provides excitatory input to the vas deferens, seminal vesicles, and prostate in males, or vaginal glands in females. In contrast, sympathetic activity causes vasoconstriction and loss of erection. The lumbar sympathetic pathway to the sexual organs originates in the thoraco-lumbar segments (T11-L2) and reaches the target organs via the corresponding sympathetic chain ganglia and the inferior mesenteric and pelvic ganglia, as in the case of the autonomic bladder control. The afferent effects of genital stimulation are conveyed centrally from somatic sensory endings via the dorsal roots of S2–S4, eventually reaching the somatic sensory cortex (reflex sexual excitation may also occur by local stimulation, as is evident in paraplegics). The reflex effects of such stimulation are increased parasympathetic activity, which, as noted, causes relaxation of the smooth muscles in the wall of the sinusoids and subsequent erection. Finally, the somatic component of reflex sexual function arises from α-motor neurons in the lumbar and sacral spinal cord segments. These neurons provide excitatory innervation to the bulbocavernosus and ischiocavernosus muscles, which are active during ejaculation in males and mediate the contractions of the perineal (pelvic floor) muscles that accompany orgasm in both male and females. Sexual functions are governed centrally by the anterior-medial and medial-tuberal zones of the hypothalamus, which contain a variety of nuclei pertinent to visceral motor control and reproductive behavior (see Box A ). Although they remain poorly understood, these nuclei act as integrative centers for sexual responses and are also thought to be involved in more complex aspects of sexuality, such as sexual preference and gender identity (see Chapter 30 ). The relevant hypothalamic nuclei receive inputs from several areas of the brain, including—as one might imagine—the cortical and subcortical structures concerned with emotion and memory (sees Chapters 29 and 31 )
- Female Reproductive System Sympathetic Innervation The sympathetic preganglionic neurons innervating the smooth muscle of the uterine wall are located in the IML at the T12-L2 level. Their preganglionic fibers pass through the sympathetic chain, exit in the lumbar splanchnic nerves, and synapse on postganglionic neurons in the inferior mesenteric ganglion. The postganglionic fibers from these neurons pass through the hypogastric plexus and innervate the female sexual organ (vagina) and the uterus (Fig. 22-10). Some preganglionic fibers from L1-L2 spinal segments descend in the sympathetic chain and synapse on postganglionic neurons in the hypogastric plexus. The postganglionic fibers from these neurons then innervate the female erectile tissue (clitoris) (Fig. 22-1). Activation of the sympathetic nervous system results in contraction of the uterus. Parasympathetic Innervation The location of the parasympathetic preganglionic neurons and the pathways they follow to innervate the uterus and female sexual organ are similar to those described for the male sexual organ (Fig. 22-10). The mechanism of vasodilation in the female erectile tissue (clitoris) is similar to that described for the male sexual organ. Parasympathetic stimulation causes stimulation of the female erectile tissue and relaxation of the uterine smooth muscle. The relaxation of the uterine smooth muscle may be variable due to hormonal influences on this muscle. The pain-sensing neurons innervating the uterus are located in the dorsal root ganglia at T12-L2 and S2-S4. Their peripheral axons pass through the hypogastric plexus and terminate in the uterus, while their central terminals synapse in the substantia gelatinosa at the T12-L2 and S2-S4 levels. The secondary pain-sensing neurons then project to the cerebral cortex via the thalamus (see Chapter 15).
- Medullary, Pontine, and Mesencephalic Control of the Autonomic Nervous System Many neuronal areas in the brain stem reticular substance and along the course of the tractus solitarius of the medulla, pons, and mesencephalon, as well as in many special nuclei (Figure 60–5), control different autonomic functions such as arterial pressure, heart rate, glandular secretion in the gastrointestinal tract, gastrointestinal peristalsis, and degree of contraction of the urinary bladder. Control of each of these is discussed at appropriate points in this text. Suffice it to point out here that the most important factors controlled in the brain stem are arterial pressure, heart rate, and respiratory rate . Indeed, transection of the brain stem above the midpontine level allows basal control of arterial pressure to continue as before but prevents its modulation by higher nervous centers such as the hypothalamus. Conversely, transection immediately below the medulla causes the arterial pressure to fall to less than one-half normal. Closely associated with the cardiovascular regulatory centers in the brain stem are the medullary and pontine centers for regulation of respiration, which are discussed in Chapter 41. Although this is not considered to be an autonomic function, it is one of the involuntary functions of the body. Control of Brain Stem Autonomic Centers by Higher Areas. Signals from the hypothalamus and even from the cerebrum can affect the activities of almost all the brain stem autonomic control centers. For instance, stimulation in appropriate areas mainly of the posterior hypothalamus can activate the medullary cardiovascular control centers strongly enough to increase arterial pressure to more than twice normal. Likewise, other hypothalamic centers control body temperature, increase or decrease salivation and gastrointestinal activity, and cause bladder emptying. To some extent, therefore, the autonomic centers in the brain stem act as relay stations for control activities initiated at higher levels of the brain, especially in the hypothalamus. In Chapters 58 and 59, it is pointed out also that many of our behavioral responses are mediated through (1) the hypothalamus, (2) the reticular areas of the brain stem, and (3) the autonomic nervous system. Indeed, some higher areas of the brain can alter function of the whole autonomic nervous system or of portions of it strongly enough to cause severe autonomic-induced disease such as peptic ulcer of the stomach or duodenum, constipation, heart palpitation, or even heart attack.
- The Hypothalamus The hypothalamus is located at the base of the forebrain, bounded by the optic chiasm rostrally and the midbrain tegmentum caudally. It forms the floor and ventral walls of the third ventricle and is continuous through the infundibular stalk with the posterior pituitary gland, as illustrated in figure A. Because of its central position in the brain and its proximity to the pituitary, it is not surprising that the hypothalamus integrates information from the forebrain, brainstem, spinal cord, and various endocrine systems, being particularly important in the central control of visceral motor functions. The hypothalamus comprises a large number of distinct nuclei, each with its own complex pattern of connections and functions. The nuclei, which are intricately interconnected, can be grouped in three longitudinal regions referred to as periventricular , medial , and lateral . They can also be grouped along the anterior—posterior dimension, which are referred to as the anterior (or preoptic), tuberal , and posterior regions (figure B). The anterior periventricular group contains the suprachiasmatic nucleus, which receives direct retinal input and drives circadian rhythms (see Chapter 28 ). More scattered neurons in the periventricular region (located along the wall of the third ventricle) manufacture peptides known as releasing or inhibiting factors that control the secretion of a variety of hormones by the anterior pituitary. The axons of these neurons project to the median eminence, a region at the junction of the hypothalamus and pituitary stalk, where the peptides are secreted into the portal circulation that supplies the anterior pituitary. Nuclei in the anterior-medial region include the paraventricular and supra- optic nuclei, which contain the neurosecretory neurons whose axons extend into the posterior pituitary. With appropriate stimulation, these neurons secrete oxytocin or vasopressin (antidiuretic hormone) directly into the bloodstream. Other neurons in the paraventricular nucleus project to the preganglionic neurons of the sympathetic and parasympathetic divisions in the brainstem and spinal cord. It is these cells that are thought to exert hypothalamic control over the visceral motor system and to modulate the activity of the poorly defined nuclei in the brainstem tegmentum that organize specific autonomic reflexes such as respiration and vomiting. The paraventricular nucleus, like other hypothalamic nuclei, receives inputs from the other hypothalamic zones, which are in turn related to the cortex, hippocampus, amygdala, and other central structures that, as noted in the text, are all capable of influencing visceral motor function. The medial-tuberal region nuclei ( tuberal refers to the tuber cinereum, the anatomical name given to the middle portion of the inferior surface of the hypothalamus) include the dorsomedial and ventromedial nuclei, which are involved in feeding, reproductive and parenting behavior, thermoregulation, and water balance. These nuclei receive inputs from structures of the limbic system, as well as from visceral sensory nuclei in the brainstem (e.g., the nucleus of the solitary tract). Finally, the lateral region of the hypothalamus is really a rostral continuation of the midbrain reticular formation. Thus, the neurons of the lateral region are not grouped into nuclei, but are scattered among the fibers of the medial forebrain bundle, which runs through the lateral hypothalamus. These cells control behavioral arousal and shifts of attention, especially as related to reproductive activities. In summary, the hypothalamus regulates an enormous range of physiological and behavioral activities, including control of body temperature, sexual activity, reproductive endocrinology, and attack-and-defense (aggressive) behavior. It is not surprising, then, that this intricate structure is the key controlling center for visceral motor activity and for homeostatic functions generally. Figure 49-11 The structure of the hypothalamus. A. Frontal view of the hypothalamus (section along the plane shown in part B). B. A medial view shows most of the main nuclei. The hypothalamus is often divided analytically into three areas in a rostocaudal direction: the preoptic area, the tuberal level, and the posterior level. The Hypothalamus Integrates Autonomic and Endocrine Functions With Behavior The hypothalamus plays a particularly important role in regulating the autonomic nervous system and was once referred to as the “head ganglion” of the autonomic nervous system. But recent studies of hypothalamic function have led to a somewhat different view. Whereas early studies found that electrical stimulation or lesions in the hypothalamus can profoundly affect autonomic function, more recent investigations have demonstrated that many of these effects are due to involvement of descending and ascending pathways of the cerebral cortex or the basal forebrain passing through the hypothalamus. Modern studies indicate that the hypothalamus functions to integrate autonomic response and endocrine function with behavior, especially behavior concerned with the basic homeostatic requirements of everyday life. The hypothalamus serves this integrative function by regulation of five basic physiological needs: It controls blood pressure and electrolyte composition by a set of regulatory mechanisms that range from control of drinking and salt appetite to the maintenance of blood osmolality and vasomotor tone. It regulates body temperature by means of activities ranging from control of metabolic thermogenesis to behaviors such as seeking a warmer or cooler environment. It controls energy metabolism by regulating feeding, digestion, and metabolic rate. It regulates reproduction through hormonal control of mating, pregnancy, and lactation. It controls emergency responses to stress, including physical and immunological responses to stress by regulating blood flow to muscle and other tissues and the secretion of adrenal stress hormones. The hypothalamus regulates these basic life processes by recourse to three main mechanisms. First, the hypothalamus has access to sensory information from virtually the entire body. It receives direct inputs from the visceral sensory system and the olfactory system, as well as the retina. The visual inputs are used by the suprachiasmatic nucleus to synchronize the internal clock mechanism to the day-night cycle in the external world (Chapter 3). Visceral somatosensory inputs carrying information about pain are relayed to the hypothalamus from the spinal and trigeminal dorsal horn (Chapters 23 and 24). In addition, the hypothalamus has internal sensory neurons that are responsive to changes in local temperature, osmolality, glucose, and sodium, to name a few examples. Finally, circulating hormones such as angiotensin II and leptin enter the hypothalamus at specialized zones along the margins of the third ventricle called circumventricular organs , where they interact directly with hypothalamic neurons. Second, the hypothalamus compares sensory information with biological set points. It compares, for example, local temperature in the preoptic area to the set point of 37°C and, if the hypothalamus is warm, activates mechanisms for heat dissipation. There are set points for a wide variety of physiological processes, including blood sugar, sodium, osmolality, and hormone levels. Finally, when the hypothalamus detects a deviation from a set point, it adjusts an array of autonomic, endocrine, and behavioral responses to restore homeostasis. If the body is too warm, the hypothalamus shifts blood flow from deep to cutaneous vascular beds and increases sweating, to increase heat loss through the skin. It increases vasopressin secretion, to conserve water for sweating. Meanwhile, the hypothalamus activates coordinated behaviors, such as seeking to change the local ambient temperature or seeking a cooler environment. All of these processes must be precisely coordinated. For example, adjustments in blood flow in different vascular beds are important for such diverse activities as thermoregulation, digestion, response to emergency, and sexual intercourse. In order to do this, the hypothalamus contains an array of specialized cell groups with different functional roles. The Hypothalamus Contains Specialized Groups of Neurons Clustered in Nuclei Although the hypothalamus is very small, occupying only about 4 grams of the total 1400 grams of adult human brain weight, it is packed with a complex array of cell groups and fiber pathways (Figure 49-11). The hypothalamus can be divided into three regions: anterior, middle, and posterior. The most anterior part of the hypothalamus, overlying the optic chiasm, is the preoptic area. The preoptic nuclei, which include the circadian pacemaker (suprachiasmatic nucleus), are mainly concerned with integration of different kinds of sensory information needed to judge deviation from physiological set point. The preoptic area controls blood pressure and composition; cycles of activity, body temperature, and many hormones; and reproductive activity. The middle third of the hypothalamus, overlying the pituitary stalk, contains the dorsomedial, ventromedial, paraventricular, supraoptic, and arcuate nuclei. The paraventricular nucleus includes both magnocellular and parvocellular neuroendocrine components controlling the posterior and anterior pituitary gland. In addition, it contains neurons that innervate both the parasympathetic and sympathetic preganglionic neurons in the medulla and the spinal cord, thus playing a major role also in regulating autonomic responses. The arcuate and periventricular nuclei, along the wall of the third ventricle, like the paraventricular nucleus contain parvocellular neuroendocrine neurons, whereas the supraoptic nucleus contains additional magnocellular neuroendocrine cells. The ventromedial and dorsomedial nuclei project mainly locally within the hypothalamus and to the periaqueductal gray matter, to regulate complex integrative functions such as control of growth, feeding, maturation, and reproduction. Finally, the posterior third of the hypothalamus includes the mammillary body and the overlying posterior hypothalamic area. In addition to the mammillary nuclei, whose function remains enigmatic, this region includes the tuberomammillary nucleus, a histaminergic cell group that is important in regulating wakefulness and arousal. The major nuclei of the hypothalamus are located for the most part in the medial part of the hypothalamus, sandwiched between two major fiber systems. A massive longitudinal fiber pathway, the medial forebrain bundle , runs through the lateral hypothalamus. The medial forebrain bundle connects the hypothalamus with the brain stem below, and with the basal forebrain, amygdala, and cerebral cortex above. Large neurons scattered among the fibers of the medial forebrain bundle provide long-ranging hypothalamic outputs that reach from the cerebral cortex to the sacral spinal cord. They are involved in organizing behaviors as well as autonomic responses. A second, smaller fiber system is located medial to the major hypothalamic nuclei, in the wall of the third ventricle. This periventricular fiber system contains longitudinal fibers that link the hypothalamus to the periaqueductal gray matter in the midbrain. This pathway is thought to be important in activating simple, stereotyped behavioral patterns, such as posturing during sexual behavior. The periventricular system also conveys the axons of the parvocellular neuroendocrine neurons located in the periventricular region, and including the paraventricular and arcuate nuclei, to the median eminence, for control of the anterior pituitary gland. They are met in the median eminence by the axons from the magnocellular neurons, which continue down the pituitary stalk to the posterior pituitary gland.
- Inputs from limbic structures. Projections from hippocampal formation through the fornix (shown in red), amygdala through the stria terminalis (shown in green), and septal area through the medial forebrain bundle (shown in blue) to the hypothalamus. Other inputs, such as those from the brainstem, are omitted from this diagram.
- Inputs from cerebral cortex. Diagrams illustrate the pathways by which the prefrontal cortex (green) and anterior cingulate gyrus (red) supply the hypothalamus by virtue of relays in the mediodorsal and midline thalamic nuclear groups (blue).
- Efferent projections of the mammillary bodies to the anterior thalamic nucleus via the mammillothalamic tract and to the midbrain tegmentum via the mammillotegmental tract.
- Major efferent projections of the hypothalamus. Not shown in this illustration are connections from the hypothalamus to the pituitary gland.
- Figure 49-12 The hypothalamus controls the pituitary gland both directly and indirectly through hormone-releasing neurons. Peptidergic neurons (5) release oxytocin or vasopressin into the general circulation through the posterior pituitary. Two general types of neurons are involved in regulation of the anterior pituitary. Peptidergic neurons (3, 4) synthesize and release hormones into the hypophyseal-portal circulation. The second type of neuron is the link between the peptidergic neurons and the rest of the brain. These neurons, some of which are monoaminergic, are believed to form synapses with peptidergic neurons either on the cell body (1) or on the axon terminal (2). The Hypothalamus Controls the Endocrine System The hypothalamus controls the endocrine system directly , by secreting neuroendocrine products into the general .979 circulation from the posterior pituitary gland, and indirectly , by secreting regulatory hormones into the local portal circulation, which drains into the blood vessels of the anterior pituitary (Figure 49-12). These regulatory hormones control the synthesis and release of anterior pituitary hormones into the general circulation. The highly fenestrated (perforated) capillaries of the posterior pituitary and median eminence of the hypothalamus facilitate the entry of hormones into the general circulation or the portal plexus. Direct and indirect control form the basis of our modern understanding of hypothalamic control of endocrine activity. Magnocellular Neurons Secrete Oxytocin and Vasopressin Directly From the Posterior Pituitary Large neurons in the paraventricular and supraoptic nuclei, constituting the magnocellular region of the hypothalamus, project to the posterior pituitary gland ( neurohypophysis ). Some of the magnocellular neuroendocrine neurons in the paraventricular and supraoptic nuclei release the neurohypophyseal hormone oxytocin, while others release vasopressin into the general circulation by way of the posterior pituitary (Figure 49-13). These peptides circulate to target organs of the body that control water balance and milk release. Oxytocin and vasopressin are peptides that contain nine amino acid residues (Table 49-2). Like other peptide hormones, they are cleaved from larger prohormones (see Chapter 15). The prohormones are synthesized in the cell body and cleaved within transport vesicles as they travel down the axons. The peptide neurophysin is a cleavage product of the processing of vasopressin and oxytocin and is released along with the hormone in the posterior pituitary. The neurophysin formed in neurons that release vasopressin differs somewhat from that produced in neurons that release oxytocin. Parvocellular Neurons Secrete Peptides That Regulate Release of Anterior Pituitary Hormones Geoffrey Harris proposed in the 1950s that the anterior pituitary gland is regulated indirectly by the hypothalamus. He demonstrated that the hypophysial portal veins, which carry blood from the hypothalamus to the anterior pituitary gland, convey important signals that control anterior pituitary secretion. In the 1970s the structure of a series of peptide hormones that carry these signals was elucidated. These hormones fall into two classes: releasing hormones and release-inhibiting hormones (Table 49-3). Of all the anterior pituitary hormones, only prolactin is under predominantly inhibitory control. Hence transection of the pituitary stalk causes insufficiency of adrenal cortex, thyroid, gonadal, and growth hormones, but increased prolactin secretion. Systematic electrical recordings have not been made from neurons that secrete releasing hormones. However, they are believed to fire in bursts because of the pulsatile nature of secretion of the anterior pituitary hormones, which show periodic surges throughout the day. Episodic firing may be particularly effective for causing hormone release and may limit receptor inactivation. The neurons that make releasing hormones are found mainly along the wall of the third ventricle. The gonadotropin-releasing hormone (GnRH) neurons tend to be located most anteriorly, along the basal part of the third ventricle. Neurons that make somatostatin, corticotropin-releasing hormone (CRH), and dopamine are located more dorsally and are found in the medial part of the paraventricular nucleus. Neurons that make growth hormone-releasing hormone (GRH), thyrotropin-releasing hormone (TRH), GnRH, and dopamine are found in the arcuate nucleus, an expansion of the periventricular gray matter that overlies the median eminence, in the floor of the third ventricle (see Figure 49-10). The median eminence contains a plexus of fine capillary loops. These are fenestrated capillaries, and the terminals of the neurons that contain releasing hormones end on these loops. The blood then flows from the median eminence into a secondary (portal) venous system, which carries it to the anterior pituitary gland (See Figure 49-11). An Overall View The three divisions of the autonomic nervous system comprise an integrated motor system that acts in parallel with the somatic motor system and is responsible for homeostasis. Esential to the functioning of the motor outflow are the visceral sensory afferents that are relayed from the nucleus of the solitary tract through a network of central autonomic control nuclei. The hypothalamus integrates somatic, visceral, and behavioral information from all of these sources, thus coordinating autonomic and endocrine outflow with behavioral state. Several features of the autonomic nervous system permit rapid integrated responses to changes in the environment. The activity of effector organs is finely controlled by coordinated and balanced excitatory and inhibitory inputs from tonically active postganglionic neurons. Moreover, the sympathetic system is greatly divergent, permitting the entire body to respond to extreme conditions In addition to the small molecule neurotransmitters— ACh and norepinephrine—a wide variety of peptides are thought to be released by autonomic neurons either onto postganglionic cells or their targets. Many of these peptides act to alter the efficacy of cholinergic or adrenergic transmission. The autonomic nervous system uses a rich variety of chemical mediators, several of which may commonly coexist in single autonomic neurons. The release of different combinations of chemical mediators from autonomic neurons may represent a means of “chemical coding” of information transfer in the different branches of the autonomic nervous system, although we are still only beginning to learn how to read the code As we shall also see in the following two chapters, the autonomic nervous system is a remarkably adaptable system of homeostatic control. It can function locally through branches of primary sensory fibers that terminate in autonomic ganglia, or intrinsically through the entire nervous system on the functions of the digestive tract. Control centers in the brain stem are involved in several autonomic reflexes. While the hypothalamus integrates behavioral and emotional responses arising from the forebrain with ongoing metabolic need to produce highly coordinated autonomic control and behavior.
- Summary of the central control of the visceral motor system. The major organizing center for visceral motor functions is the hypothalamus (see Box A ). Central Control of the Visceral Motor Functions The visceral motor system is regulated in part by circuitry in the cerebral cortex: Involuntary visceral reactions such as blushing in response to consciously embarrassing stimuli, vasoconstriction and pallor in response to fear, and autonomic responses to sexual situations make this plain. Indeed, autonomic function is intimately related to emotional experience and expression, as described in Chapter 29 . In addition, the hippocampus, thalamus, basal ganglia, cerebellum, and reticular formation all influence the visceral motor system. The major center in the control of the visceral motor system, however, is the hypothalamus ( Box A ). The hypothalamic nuclei relevant to visceral motor function project to the nuclei in the brainstem that organize many visceral reflexes (e.g., respiration, vomiting, urination), to the cranial nerve nuclei that contain parasympathetic preganglionic neurons, and to the sympathetic and parasympathetic preganglionic neurons in the spinal cord. The general organization of this central autonomic control is summarized in Figure 21.6 , and some important clinical manifestations of damage to this descending system are illustrated in Box B . Although the hypothalamus is the key structure in the overall organization of visceral function, and in homeostasis generally, the visceral motor systemcontinues to function independently if disease or injury impedes the influence of this controlling center. The major subcortical centers for the ongoing regulation of the autonomic function in the absence of hypothalamic control are a series of poorly understood nuclei in the brainstem tegmentum that organize specific visceral functions such as cardiac reflexes, reflexes that control the bladder, and reflexes related to sexual function, as well as other critical autonomic reflexes such as respiration and vomiting. The afferent information from the viscera that drives these brainstem centers is, as noted already, received by neurons in the nucleus of the solitary tract, which relays these signals to the hypothalamus and to the various autonomic centers in the brainstem tegmentum.
- Figure 51-1 Homeostatic processes can be analyzed in terms of control systems. A. A control system regulates a controlled variable. When a feedback signal indicates the controlled variable is below or above the set point an error signal is generated. This signal turns on (or facilitates) appropriate behaviors and physiological responses, and turns off (or suppresses) incompatible responses. An error signal also can be generated by external (incentive) stimuli. B. A negative feedback system without a set point controls fat stores. (Based on data of DiGirolamo and Rudman 1968.) SO FAR IN THIS BOOK OUR discussion of the neural control of behavior has focused on how the brain translates external sensory information about events in the environment into coherent perceptions and motor action. In the final two parts of the book we examine how development and learning profoundly shape the brain's ability to do this. These parts of the book are to a large degree concerned with the cognitive aspects of behavior—what a person knows about the outside world. However, behavior also has non cognitive aspects that reflect not what the individual knows but what he or she needs or wants. Here we are concerned with how individuals respond to internal rather than external stimuli. This is the domain of motivation. Motivation is a catch-all term that refers to a variety of neuronal and physiological factors that initiate, sustain, and direct behavior. These internal factors are thought to explain, in part, variation in the behavior of an individual over time. As discussed earlier in this book, the behaviorists who dominated the study of behavior in the first half of this century largely ignored internal factors in their attempts to explain behavior. With the rise of cognitive psychology a few decades ago the behaviorist paradigm has receded and motivation, with all of its complexity, has become the subject of serious scientific study once again. The biological study of motivation has until quite recently been confined to studies of simple physiological or homeostatic instances of motivation called drive states. For this reason our discussion here focuses primarily on drive states, which are the outcome of homeostatic processes related to hunger, thirst, and temperature regulation. Drive states are characterized by tension and discomfort due to a physiological need followed by relief when the need is satisfied. It is important to recognize, however, that drive states are merely one subtype, perhaps the simplest examples, of the motivational states that direct behavior. In general, motivational states may be broadly classified into two types: (1) elementary drive states and more complex physiological regulatory forces brought into play by alterations in internal physical conditions such as hunger, thirst, and temperature, and (2) personal or social aspirations acquired by experience. Freud and contemporary cognitive psychologists have suggested that both forms, but especially personal and social aspirations, represent a complex interplay between physiological and social forces, and between conscious and unconscious mental processes. The neurobiological study of the second type of motivational states is in its infancy. The issues that surround drive states relate to survival. Activities that enhance immediate survival, such as eating or drinking, or those that ensure long-term survival, such as sexual behavior or caring for offspring, are pleasurable and there is a great natural urge to repeat these behaviors. Drive states steer behavior toward specific positive goals and away from negative ones. In addition, drive states require organization of individual behaviors into a goal-oriented sequence. Attainment of the goal decreases the intensity of the drive state and thus the motivated behavior ceases. A hungry cat is ever alert for the occasional mouse, ready to pounce when it comes into sight. Once satiated, the cat will not pounce again for some time. Finally, drive states have general effects; they increase our general level of arousal and thereby enhance our ability to act. Drive states therefore serve three functions: they direct behavior toward or away from a specific goal; they organize individual behaviors into a coherent, goal-oriented sequence; and they increase general alertness, energizing the individual to act. The drive states that neurobiologists have studied most effectively are those related to temperature regulation, hunger, and thirst. Until recently, these drive states were inferred from behavior alone. But as we learn more about the physiological correlates of drive states, we rely less on traditional psychological concepts of motivation and more on concepts derived from servo-control models applied to living organisms. Admittedly, such an approach reduces drive states to a complex homeostatic reflex that is responsive to multiple stimuli. Some of these stimuli are internal in response to tissue deficits; others are external (eg, the sight or smell of food) and are regulated by excitatory and inhibitory systems. Since regulation of internal states involves the autonomic nervous system and the endocrine system, we shall consider the relationship of motivational states to autonomic and neuroendocrine responses. We first examine how servo-control models have made the study of drive states amenable to biological experimentation. We then examine the regulation of these simple motivational states by factors other than tissue deficits, such as circadian rhythms, ecological constraints, and pleasure. Finally, we discuss the neural systems of the brain concerned with reward or reinforcement, an important component of motivation. These neural systems have been well delineated. Most addictive drugs, such as nicotine, alcohol, opiates, and cocaine, produce their actions by acting on or co-opting the same neural pathways that mediate positively motivated behaviors essential for survival. Drive States Are Simple Cases of Motivational States That Can Be Modeled as Servo-Control Systems Drive states can be understood by analogy with control systems, or servomechanisms , that regulate machines. While specific physiological servomechanisms have not yet been demonstrated directly, the servomechanism model permits us to organize our thinking about the complex operation of homeostasis, and makes it possible to define experimentally the physiological control of homeostasis. This approach has been most successfully applied to temperature regulation. Because body temperature can be readily measured, the mechanism regulating temperature has been studied by examining the relationship between the internal stimulus (temperature) and various external stimuli. This control system approach has been less successful when applied to more complex regulatory behaviors, such as feeding, drinking, and sex, in which the relevant internal stimuli are difficult to identify and measure. Nevertheless, at present, the control systems model is probably the best approach to analyzing even these more complex internal states. Servomechanisms maintain a controlled variable within a certain range. One way of regulating the controlled variable is to measure it by means of a feedback detector and compare the measured value with a desired value, or set point. The comparison is accomplished by an error detector, or integrator , that generates an error signal when the value of the controlled variable does not match the set point. The error signal then drives controlling elements that adjust the controlled system in the desired direction. The error signal is controlled not only by internal feedback stimuli but also by external stimuli. All examples of physiological control seem to involve both inhibitory and excitatory effects, which function together to adjust the control system (Figure 51-1). The control system used to heat a home illustrates these principles. The furnace system is the controlling element. The room temperature is the controlled variable. The home thermostat is the error detector. The setting on the thermostat is the set point. Finally, the output of the thermostat is the error signal that turns the control element on or off.
- Figure 51-2 This sagittal section of the human brain illustrates the hypothalamic regions concerned with heat conservation and heat dissipation. Temperature Regulation Involves Integration of Autonomic, Endocrine, and Skeletomotor Responses Temperature regulation nicely fits the model of a control system. Normal body temperature is the set point in the system of temperature regulation. The integrator and many controlling elements for temperature regulation appear to be located in the hypothalamus. Because temperature regulation requires integrated autonomic, endocrine, and skeletomotor responses, the anatomical connections of the hypothalamus make this structure well suited for this task. The feedback detectors collect information about body temperature from two main sources: peripheral temperature receptors located throughout the body (in the skin, spinal cord, and viscera) and central receptors located mainly in the hypothalamus. The detectors of temperature, both low and high, are located only in the anterior hypothalamus. The hypothalamic receptors are probably neurons whose firing rate is highly dependent on local temperature, which in turn is importantly affected by the temperature of the blood. Although the anterior hypothalamic area is involved in temperature sensing, control of body temperature appears to be regulated by separate regions of the hypothalamus. The anterior hypothalamus mediates decreases and the posterior hypothalamus (preoptic area) mediates increases in body temperature. Thus, electrical stimulation of the anterior hypothalamus causes dilation of blood vessels in the skin, panting, and a suppression of shivering, responses that decrease body temperature. In contrast, electrical stimulation of the posterior hypothalamus produces an opposing set of responses that generate or conserve heat (Figure 51-2). As with fear responses, which are evoked by electrical stimulation of the hypothalamus (Chapter 50), temperature regulatory responses evoked by electrical stimulation also include appropriate nonvoluntary responses involving the skeletomotor system. For example, stimulation of the anterior hypothalamus (preoptic area) produces panting, while stimulation of the posterior hypothalamus produces shivering. Ablation experiments corroborate the critical role of the hypothalamus in regulating temperature. Lesions of the anterior hypothalamus cause chronic hyperthermia and eliminate the major responses that normally dissipate excess heat. Lesions in the posterior hypothalamus have relatively little effect if the animal is kept at room temperature (approximately 22°C). If the animal is exposed to cold, however, it quickly becomes hypothermic because the homeostatic mechanisms fail to generate and conserve heat.
- Figure 51-3 Peripheral and central information on temperature is summated in the hypothalamus. Changes in either room temperature or local hypothalamic temperature alter the response rate of rats trained to press a button to receive a brief burst of cool air. When the room temperature is increased, thus presumably increasing skin temperature, the response rate increases roughly in proportion to the temperature increase (points a and b). If the temperature of the hypothalamus is also increased (by perfusing warm water through a hollow probe), the response rate reflects a summation of information on skin temperature and hypothalamic temperature (points c and d). If the skin temperature remains high enough but the hypothalamus is cooled, the response rate decreases or is suppressed altogether (point e). (From data of Corbit 1973 and Satinoff 1964.) The hypothalamus also controls endocrine responses to temperature challenges. Thus, long-term exposure to cold can enhance the release of thyroxine, which increases body heat by increasing tissue metabolism. In addition to driving appropriate autonomic, endocrine, and nonvoluntary skeletal responses, the error signal of the temperature control system can also drive voluntary behaviors that minimize the error signal. For example, a rat can be taught to press a button to receive puffs of cool air in a hot environment. After training, when the chamber is at normal room temperature, the rat will not press the button for cool air. If the anterior hypothalamus is locally warmed by perfusing it with warm water through a hollow probe, the rat will run to the cool-air button and press it repeatedly. Hypothalamic integration of peripheral and central inputs can be demonstrated by heating the environment (and thereby the skin of the animal) and concurrently cooling or heating the hypothalamus. When both the environment and hypothalamus are heated, the rat presses the cool-air button faster than when either one is heated alone. However, even in a hot environment the pressing of the button for cool air can be suppressed completely by cooling the hypothalamus (Figure 51-3). Recordings from neurons in the preoptic area and the anterior hypothalamus support the idea that the hypothalamus integrates peripheral and central information relevant to temperature regulation. Neurons in this region, called warm-sensitive neurons , increase their firing when the local hypothalamic tissue is warmed. Other neurons, called cold-sensitive neurons , respond to local cooling. The warm-sensitive neurons, in addition to responding to local warming of the brain, are usually excited by warming the skin or spinal cord and are inhibited by cooling the skin or spinal cord. The cold-sensitive neurons exhibit the opposite behavior. Thus, these neurons could integrate the thermal information from peripheral receptors with that from neurons within the brain. Furthermore, many temperature-sensitive neurons in the hypothalamus also respond to nonthermal stimuli, such as osmolarity, glucose, sex steroids, and blood pressure. In humans the set point of the temperature control system is approximately 98.6°F (37°C), although it normally varies somewhat diurnally, decreasing to a minimum during sleep. The set point can be altered by pathological states, for example by the action of pyrogens, which induce fever. Systemic pyrogens, such as the macrophage product interleukin-1, enter the brain at regions in which the blood-brain barrier is incomplete, such as the preoptic area, and act there to increase the set point. The body temperature then rises until the new set point is reached. When this occurs a part of the brain known as the antipyretic area is activated and limits the magnitude of the fever. The antipyretic area includes the septal nuclei, which are located anterior to the hypothalamic preoptic areas, near the anterior commissure. The antipyretic area is innervated by neurons that use the peptide vasopressin as transmitter. Injection of vasopressin into the septal area counteracts fever in a manner similar to that of antipyretic drugs, such as aspirin and indomethacin, suggesting that some of the effects of these drugs are mediated by the central release of vasopressin. The antipyretic action of aspirin and indomethacin is blocked by injection into the septal nuclei of a vasopressin antagonist. In fact, convulsions brought on by high fevers may in part be evoked by vasopressin released in the brain as part of the antipyretic response. The control of body temperature is a clear example of the integrative action of the hypothalamus in regulating autonomic, endocrine, and drive-state control. It illustrates how the hypothalamus operates both directly on the internal environment and indirectly, by providing information about the internal environment to higher neural systems.
- Figure 51-4 Animals tend to adjust their food intake to achieve a normal body weight. The plots show a schematized growth curve for a group of rats. At arrow 1 one-third of the animals were maintained on their normal diet (curve b), one-third were force-fed (curve a), and one-third were placed on a restricted diet (curve c). At arrow 2 all rats were placed on a normal (ad libitum) diet. The force-fed animals lost weight and the starved animals gained weight until the mean weight of the two groups approached that of the normal growth curve (b). (Adapted from Keesey et al. 1976.) Chapter 51 / Motivational and Addictive States Feeding Behavior Is Regulated by a Variety of Mechanisms Like temperature regulation, feeding behavior may also be analyzed as a control system, although at every level of analysis the understanding of feeding is less complete. One reason for thinking that feeding behavior is subject to a control system is that body weight seems to be regulated by a set point. Humans often maintain the same body weight for many years. Since even a small increase or decrease of daily caloric intake could eventually result in a substantial weight change, the body must be governed by feedback signals that control nutrient intake and metabolism. Control of nutrient intake is seen most clearly in animals in which body weight is altered from the set point by food deprivation or force-feeding. In both instances the animal will adjust its subsequent food intake (either up or down) until it regains a weight appropriate for its age (Figure 51-4). Animals are thus said to defend their body weight against perturbations. Whereas body temperature is remarkably similar from one individual to another, body weight varies greatly. Furthermore, the apparent set point of an individual can vary with stress, palatability of the food, exercise, and many other environmental and genetic factors. One possible explanation for this difference between regulation of temperature and body weight is that the set point for body weight can itself be changed by a variety of factors. Another possibility is that body weight is regulated by a control system that has no formal set-point mechanism but which nevertheless functions as if there were a set point (Figure 51-1B).
- Figure 51-5 The set point for body weight appears to be altered by lesions of the lateral hypothalamus. Three groups of rats were used in this experiment. The control group was maintained on a normal diet. On day 0 the animals of the other two groups received small lesions in the lateral hypothalamus. One of these groups had been maintained on a normal diet; the other group had been starved before the lesion and consequently had lost body weight. After the lesion all animals were given free access to food. The lesioned animals that had not been prestarved initially decreased their food intake and lost body weight, while those that were prestarved rapidly gained weight until they reached the level of the other lesioned animals. (Adapted from Keesey et al. 1976.) Dual Controlling Elements in the Hypothalamus Contribute to the Control of Food Intake Food intake is thought to be under the control of two regions in the hypothalamus: a ventromedial region and a lateral region. In 1942 Albert W. Hetherington and Stephen Walter Ranson discovered that destruction of the ventromedial nuclei of the hypothalamus produces overeating ( hyperphagia ) and severe obesity. In contrast, bilateral lesions of the lateral hypothalamus produce severe neglect of eating ( aphagia ) so that the animal dies unless force-fed. Electrical stimulation produces the opposite effects of lesions. Whereas stimulation of the ventromedial region suppresses feeding, stimulation of the lateral hypothalamus elicits feeding. These observations were originally interpreted to mean that the lateral hypothalamus contains a feeding center and the medial hypothalamus a satiety center. This conclusion was reinforced by studies showing that chemical stimulation of these parts of the hypothalamus can also alter feeding behavior. This conceptually attractive conclusion proved faulty, however, as it became clear that the brain is not organized into discrete centers that by themselves control specific functions. Rather, as with perception and action, the neural circuits mediating homeostatic functions such as feeding are distributed among several structures in the brain. The effects of lateral or medial hypothalamic lesions on feeding are thought to be due in part to dysfunctions that result from damage to other structures. Three factors are particularly important: (1) alteration of sensory information, (2) alteration of set point, and (3) interference with behavioral arousal because of damage to dopaminergic fibers of passage. First, lateral hypothalamic lesions sometimes result in sensory and motor deficits as a result of the destruction of fibers of the trigeminal system and the dopaminergic fibers of the medial forebrain bundle. The sensory loss can contribute to the loss of feeding as well as to the so-called sensory neglect seen after lateral hypothalamic lesions. Thus, a unilateral lesion of the lateral hypothalamus results in loss of orienting responses to visual, olfactory, and somatic sensory stimuli presented contralateral to the lesion. Similarly, feeding responses to food presented contralaterally are also diminished. It is not clear whether this sensory neglect is due to disruption of sensory systems or to interference with motor systems directing responses contralateral to the lesion. Altered sensory responses are also seen in animals with lesions in the region of the ventromedial nucleus. These animals have heightened responses to the aversive or attractive properties of food and other stimuli. On a normal diet they eat more than do animals without lesions. Since the reduction in eating is similar to that seen in normal animals that are made obese by force-feeding, the enhanced sensory responsiveness to food of animals with ventromedial hypothalamic lesions is, at least in part, a consequence rather than a cause of the obesity. This interpretation is supported by Stanley Schachter's finding that some obese humans with no evidence of damage to the region of the ventromedial hypothalamus are also unusually responsive to the taste of food. Second, Shypothalamic lesions may alter the set point for regulating body weight. Rats that were starved to reduce their weight before a small lesion was made in the lateral hypothalamus ate more than normal amounts and gained weight when they resumed eating, whereas the controls (nonstarved) lost weight (Figure 51-5). The starvation apparently brings the weight of these animals below the set point determined by the lateral lesion. Conversely, animals that were force-fed before ventromedial hypothalamic lesions did not overeat, which they would have done if they had not been previously force-fed. Third, lesions of the lateral hypothalamus can damage dopaminergic fibers that course from the substantia nigra to the striatum via the medial forebrain bundle as well as those that emanate from the ventral tegmental area (the mesolimbic projections) and innervate structures associated with the limbic system (the prefrontal cortex, amygdala, and nucleus accumbens; see Chapter 45). When nigrostriatal dopaminergic fibers are experimentally sectioned bilaterally below or above the level of the hypothalamus or are destroyed by a specific toxin 6-OH dopamine, animals exhibit a hypoarousal state and life-threatening aphagia similar to that observed after lateral hypothalamic lesions. The loss of dopamine does not account entirely for the lateral hypothalamus syndrome. The physiological profile and recovery of eating patterns are different after lesions of the lateral hypothalamus and depletion of dopamine, demonstrating that both the dopamine system and hypothalamic substrates contribute to the control of feeding. Lesioning of dopaminergic neurons alone or loss of the neurons of the lateral hypothalamus alone (using the excitotoxins kainic or ibotenic acid) produces less severe behavioral deficits than those seen after the classical lateral hypothalamus lesions. The combined loss of lateral hypothalamic neurons and dopaminergic fibers results in the classical syndrome by impairing both the substrate for monitoring physiological feedback and the neural systems that generate appropriate behavior. In fact, the dopamine agonist apomorphine restores eating and drinking responses to physiological challenges in rats after depletion of dopamine, but not in rats with lateral hypothalamic lesions. Below we shall examine the role of dopamine in food reward and reinforcement more generally when we consider studies of intracranial self-stimulation, the effect of dopamine-blocking drugs on learned behavior to obtain food, and the reinforcing effects of drugs of addiction. Some of the strongest evidence implicating the hypothalamus in the control of feeding comes from studies showing that a wide spectrum of transmitters produces profound alterations of feeding behavior when injected into the lateral hypothalamus and the area of the paraventricular nuclei. These studies also illustrate that different chemical systems are involved in the control of different classes of nutrients. Application of norepinephrine to the paraventricular nucleus greatly stimulates feeding; but, if given a choice, animals will eat more carbohydrate than protein or fat. In contrast, application of the peptide galanin selectively increases ingestion of fat whereas opiates enhance consumption of protein.
- Figure 51-6 Hypothetical model of the mechanisms that regulate energy balance in mammals. (Adapted from Hervey 1969.) Food Intake Is Controlled by Short-Term and Long-Term Cues What cues does an organism use to regulate feeding? Two main cues for hunger have been identified: short-term cues that regulate the size of individual meals and long-term cues that regulate overall body weight (Figure 51-6). Short-term cues consist primarily of chemical properties of the food that act in the mouth to stimulate feeding behavior and in the gastrointestinal system and liver to inhibit feeding. The short-term satiety signals impinge on the hypothalamus through visceral afferent pathways, communicating primarily with the lateral hypothalamic regions. The effectiveness of short-term cues is modulated by long-term signals that reflect body weight. As we shall discuss in greater detail below, one such important signal is the peptide leptin, which is secreted from fat storage cells ( adipocytes ). By means of this signal, body weight is kept reasonably constant over a broad range of activity and diet. Daily energy expenditure is remarkably consistent when expressed as a function of body size (Figure 51-7A). Body weight is also maintained at a set level by self-regulating feedback mechanisms that adjust metabolic rate when the organism drifts away from its characteristic set point (Figure 51-7B). An animal maintained on a reduced-calorie diet eventually needs less food to maintain its weight because its metabolic rate decreases. Several humoral signals are thought to be important for short-term regulation of feeding behavior. The hypothalamus has glucoreceptors that respond to blood glucose levels. This system probably stimulates feeding behavior (in contrast to autonomic responses to blood glucose) primarily during emergency states in which blood glucose falls drastically. In addition, gut hormones released during a meal may contribute to satiety. Considerable evidence for such a humoral short-term signal comes from studies of the peptide cholecystokinin. Cholecystokinin is released from the duodenum and upper intestine when amino acids and fatty acids are present in the tract. Cholecystokinin released in the gut acts on visceral afferents that affect brain stem and hypothalamic areas, which are themselves sensitive to cholecystokinin. Injection into the ventricles or specifically into the paraventricular nucleus of small quantities of cholecystokinin and several other peptides (including neurotensin, calcitonin, and glucagon) also inhibits feeding. Therefore, chole-cystokinin released as a neuropeptide in the brain may also inhibit feeding, independently of its release from the gut Cholecystokinin is an example of a hormone or neuromodulator that has independent central and peripheral actions that are functionally related. Other examples include luteinizing hormone-releasing hormone (sexual behavior), adrenocorticotropin (stress and avoidance behavior), and angiotensin (responses to hemorrhage). The use of the same chemical signal for related central and peripheral functions is widespread in both vertebrates and invertebrates. Certain invertebrates, such as the sea slug Aplysia , have specific serotonergic neurons that both enhance feeding responses (by acting directly on muscles involved in consuming food) and promote arousal (by enhancing the excitability of central motor neurons that innervate these same muscles).
- The brain integrates multiple peripheral and neural signals to control the regulation of energy homeostasis, maintaining a balance between food intake and energy expenditure. Peripheral factors indicative of long-term energy status are produced by adipose tissue (leptin, adiponectin) and the pancreas (insulin), whereas the acute hunger signal ghrelin (produced in the stomach), and satiety signals such as the gut hormones peptide YY(3–36) (PYY(3–36)), pancreatic polypeptide (PP), amylin and oxyntomodulin (OXM) indicate near-term energy status. The incretin hormones glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and potentially OXM improve the response of the endocrine pancreas to absorbed nutrients. Further feedback is provided by nutrient receptors in the upper small bowel, and neural signals indicating distention of the stomach's stretch receptors, which are primarily conveyed by the vagal afferent and sympathetic nerves to the nucleus of the solitary tract (NTS) in the brain stem. The arcuate nucleus (ARC) of the hypothalamus, which is located between the third ventricle and the median eminence, integrates these energy homeostatic feedback mechanisms. It accesses the short- and long-term hormonal and nutrient signals from the periphery via semi-permeable capillaries in the underlying median eminence, and receives neuronal feedback from the NTS. These collated signals act on two distinct subsets of neurons that control food intake in the ARC, which act as an accelerator and a brake respectively. The first subset co-expresses the orexigenic (appetite stimulating) agouti-related peptide (AgRP) and neuropeptide Y (NPY) neurotransmitters, acting as an accelerator in the brain to stimulate feeding. The other neuronal population releases the anorexigenic cocaine- and amphetamine -regulated transcript (CART) and pro-opiomelanocortin (POMC) neurotransmitters, both of which inhibit feeding. Both neuronal populations innervate the paraventricular nucleus (PVN), which, in turn, sends signals to other areas of the brain. These include hypothalamic areas such as the ventromedial nucleus, dorsomedial nucleus and the lateral hypothalamic area, which modulate this control system. Neural brain circuits integrate information from the NTS and multiple hypothalamic nuclei to regulate overall body homeostasis.
- Drinking Is Regulated by Tissue Osmolality and Vascular Volume The hypothalamus regulates water balance by its control of hormones, such as antidiuretic hormone. The hypothalamus also regulates aspects of drinking behavior. Unlike feeding, where intake is critical, the amount of water taken in is relatively unimportant as long as the minimum requirement is met. Within broad limits, excess intake is readily eliminated by the kidney. Nevertheless, a set point, or ideal amount of water intake, appears to exist, since too much or too little drinking represents inefficient behavior. If an animal takes in too little liquid at one time, it must soon interrupt other activities and resume its liquid intake to avoid underhydration. Likewise, drinking a large amount at one time results in unneeded time spent drinking as well as urinating to eliminate the excess fluid. Drinking is controlled by two main physiological variables: tissue osmolality and vascular (fluid) volume. This has led Alan Epstein to propose that the principal inputs controlling thirst arise when both physiological variables are depleted ( double depletion hypothesis ). Signals related to the variables reach mechanisms in the brain that control drinking either through afferent fibers from peripheral receptors or by humoral actions on receptors in the brain itself. These inputs control the physiological mechanisms of water conservation in such a way that fluid intake is coordinated with the control of fluid loss so as to maintain water balance. Thus, the hypothalamus integrates hormonal and osmotic cues sensing cell volume and the state of the extracellular space. The volume of water in the intracellular compartment is normally approximately double that of the extracellular space. This delicate balance is determined by the osmotic equilibrium between the compartments, which in turn is determined by extracellular sodium. The control of sodium is therefore a key element in the homeostatic mechanism regulating thirst. The two drives, thirst and salt appetite, appear to be handled by separate but interrelated mechanisms. Drinking also can be controlled by dryness of the tongue. Hyperthermia, detected at least in part by thermosensitive neurons in the anterior hypothalamus, may also contribute to thirst. The feedback signals for water regulation derive from many sources. Osmotic stimuli can act directly on osmoreceptor cells (or receptors that sense the level of Na+), probably neurons, in the hypothalamus. The feedback signals for vascular volume are located in the low-pressure side of the circulation—the right atrium and adjacent walls of the great veins—and large volume changes may also affect arterial baroreceptors in the aortic arch and carotid sinus. Signals from these sources can initiate drinking. Low blood volume, as well as other conditions that decrease body sodium, also results in increased renin secretion from the kidney. Renin, a proteolytic enzyme, cleaves plasma angiotensinogen into angiotensin I, which is then hydrolyzed to the highly active octapeptide angiotensin II. Angiotensin II elicits drinking as well as three other physiological actions that compensate for water loss: vasoconstriction, increased release of aldosterone, and increased release of vasopressin For blood-borne angiotensin to affect behavior it must pass through the blood-brain barrier at specialized regions of the brain. The subfornical organ is a small neuronal structure that extends into the third ventricle and has fenestrated capillaries that readily permit the passage of blood-borne molecules (see Appendix B on the blood-brain barrier). The subfornical organ is sensitive to low concentrations of angiotensin II in the blood, and this information is conveyed to the hypothalamus by a neural pathway between the subfornical organ and the preoptic area. Neurons in this pathway in turn use an angiotensin-like molecule as a transmitter. Thus the same molecule regulates drinking by functioning as a hormone and a neurotransmitter. The preoptic area also receives information from baroreceptors throughout the body. This information is conveyed to various brain structures that initiate a search for water and drinking. Information from baroreceptors is also sent to the paraventricular nucleus, which mediates the release of vasopressin, which in turn regulates water retention. The signals that terminate drinking are less well understood than those that initiate drinking. It is clear, however, that the termination signal is not always merely the absence of the initiating signal. This principle holds for many examples of physiological and behavioral regulation, including feeding. Thus, for example, drinking initiated by low vascular fluid volume (eg, after severe hemorrhage) terminates well before the deficit is rectified. This is highly adaptive since it prevents water intoxication from excessive dilution of extracellular fluids and seems to prevent overhydration that could result from absorption of fluid in the alimentary system long after the cessation of drinking.
