Amalgum I
- 1. Dental Amalgam Dr. Hala Bahgat Dr. Dina Mostafa
- 2. Amalgam: Any alloy + Mercury (Hg) Dental Amalgam: Dental alloy + Mercury (Hg) Alloy for Dental Amalgam : Alloy powder used in dental amalgam Ag , Sn , Cu and Zn Amalgamation reaction: Chemical reaction between dental alloy and mercury at room Dr. Hala Bahgat temperature Dr. Dina Mostafa
- 3. Dental Amalgam Mode of supply Hg Powde r Dr. Hala Bahgat Dr. Dina Mostafa
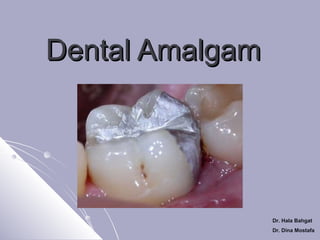
- 4. Dental Amalgam Posterior Direct Filling Materials USES 1. Better in small occlusal cavity Class I 2. Class II with some precautions Dr. Hala Bahgat Dr. Dina Mostafa
- 5. 3. Core material for crown build up Dr. Hala Bahgat Dr. Dina Mostafa
- 6. Advantages: • Ease of manipulation • High compressive strength • Low cost • Marginal leakage decreases with time • Long clinical service life Disadvantages: • Metallic color • Brittle and low toughness • Thermal irritation •Subject to corrosion and galvanic action • subject to creep • Does not bond to tooth structure Dr. Hala Bahgat Dr. Dina Mostafa
- 7. Dr. Hala Bahgat Dr. Dina Mostafa
- 8. Silver (Ag) 1. 2. 3. 4. Increases strength and hardness. Increases setting expansion. Increases corrosion resistance. Decreases creep. Tin (Sn) 1. Decreases strength and hardness. 2. Decreases setting expansion (causes contraction). 3. Decreases corrosion resistance. 4. Increases creep. It is a time dependent deformation of a material under its own weight (below P.L.), having its melting temperature near the ambient temperature. Dr. Hala Bahgat Dr. Dina Mostafa
- 9. 73.2-80% Ag 20-26.8% Sn Below 480°C γ Dr. Hala Bahgat Dr. Dina Mostafa
- 10. Silver 1.Increases strength and hardness. 2.Increases setting expansion. 3.Increase corrosion resistance. 4.Decreases creep. Tin (Sn) 1. Decreases strength and hardness. 2. Decreases setting expansion (causes contraction). 3. Decreases corrosion resistance. 4. Increases creep. 5. Formation of intermetallic compound with silver. 6. Increases the rate of amalgamation. 7. Increases plasticity of amalgam mass during Dr. Hala Bahgat condensation. Dr. Dina Mostafa
- 11. Copper (Cu) 1.Increases strength and hardness. 2.Increases setting expansion. 3.Increases corrosion resistance. 4.Decreases creep. Zinc (Zn) 1.Deoxidizer (scavenger) during the alloy manufacturing. 2.Increases strength. 3.Increases setting expansion. 4.Increases plasticity of amalgam mass during condensation. Dr. Hala Bahgat Dr. Dina Mostafa
- 12. + Mercury Hg 1.Decreases strength and hardness. 2.Highly increases the setting expansion. 3.Decreases corrosion resistance. 4.Increases creep. Dr. Hala Bahgat Dr. Dina Mostafa
- 13. Classification of Dental Amalgam I- According to Zinc Content Zinc containing amalgams More than 0.01- 2% mx Zinc free amalgams Less than 0.01% Dr. Hala Bahgat Dr. Dina Mostafa
- 14. II- According to Particles Shape Spherical Lathe Cut Spheroidal Advantage of spherical particles compared to lathe cut: 1- Lower surface area/volume 2- They take less mercury 3- Need less condensation pressure but larger condenser 4- Fast setting 5-They produce smoother surface 6- They offer superior strength property Dr. Hala Bahgat Dr. Dina Mostafa
- 15. III- According to Particles Size Coarse Cut Fine Cut Microfine Cut 100 – 200 µm 15 – 35 µm Less than 3 µm Advantage of fine cut particles compared to coarce cut: Disadvantages: Increase total area/volume 1- More consumption of mercury 2- Fast setting Will need more Hg 3- They produce smoother surface 4- They offer superior strength property 5- Better adaptation to cavity walls Less mechanical properties Dr. Hala Bahgat Dr. Dina Mostafa
- 16. IV- According to Copper Content High Copper Amalgams Low Copper Amalgams or Conventional Amalgams Admixed amalgams Less than 6% Cu Unicompositional amalgams More than 6% Cu Dr. Hala Bahgat Dr. Dina Mostafa
- 17. Low Copper or Conventional Amalgam Composition Silver (Ag) 65% Tin (Sn) Lathe coarse cut 29% Copper (Cu) Zinc (Zn) 6% mx 2% Dr. Hala Bahgat Dr. Dina Mostafa
- 18. Theory of Setting Diffusion Theory: Wetting Diffusion Surface reaction Ag2Hg3 (γ 1) Sn8Hg (γ 2) Hg γ Hg Dr. Hala Bahgat Dr. Dina Mostafa
- 19. Ag3 Sn + Hg Ag2Hg3 (γ) (γ 1) + Sn8Hg + Ag3Sn + voids (γ 2) unreacted (γ) Ag3Sn (γ ) Ag2Hg3 (γ 1) Sn8Hg (γ 2) voids Strength Hg: Setting expansion Corrosion resistance Creep Dr. Hala Bahgat Dr. Dina Mostafa
- 20. Ag3Sn (γ ) Ag2Hg3 (γ 1) Sn8Hg (γ 2) voids Dr. Hala Bahgat Dr. Dina Mostafa
- 21. High Copper Dental Amalgams γ 2 Free Amalgams More than 6% Cu Admixed amalgams Unicompositional amalgams Dr. Hala Bahgat Dr. Dina Mostafa
- 22. Admixed Dental Amalgams (9-20% Cu) Lathe coarse cut Spherical cut AgCu + 50-70% 30-50% Intermetallic alloy (lathe cut) Eutectic alloy (spherical) 72%Ag 28%Cu 779.4°C Ag3Sn (γ) Dr. Hala Bahgat Dr. Dina Mostafa
- 23. Setting Reactions of Admixed Amalgam 1- Dissolution of mercury in the alloy powder [Ag3Sn+ Ag - Cu] + Hg → Ag2Hg3 γ (eutectic) (γ ) + Sn8Hg + [Ag3Sn+ Ag - Cu] + voids (γ 2) 1 Ag3Sn Cu6Sn5 (γ ) Ag2Hg3 (γ 1) AgCu Sn8Hg (γ 2) voids 2- Elimination of γ 2 (Solid State Reaction) Ag – Cu + Sn8Hg (γ 2) Cu6Sn5 + Ag2Hg3 + Ag-cu unreacted + voids η (γ 1) (eutectic) Dr. Hala Bahgat Dr. Dina Mostafa
- 24. Ag3Sn (γ ) Ag2Hg3 Cu6Sn5 (γ 1) (η) AgCu voids Dr. Hala Bahgat Dr. Dina Mostafa
- 25. Ag3Sn Cu6Sn5 (γ ) Ag2Hg3 (γ 1) AgCu Sn8Hg (γ 2) voids Disadvantages: 1- Sedimentation of one type of the particles 2- Surface oxidation of the silver - copper eutectic particles during storage Dr. Hala Bahgat Dr. Dina Mostafa
- 26. Unicompositional Dental Amalgams Composition: Silver Tin Copper 13- 30%. 40-60%. 27 - 30%~ Manufacturing of the powder: The powder is produced by melting and atomization of the liquid alloy to produce spherical powder particles. Each unicompositional spherical powder particle is composed of γ Ag3Sn phase and ε Cu3Sn (epsilon) phase which is concentrated near the particle surface. Ag3Sn Cu3Sn Dr. Hala Bahgat Dr. Dina Mostafa
- 27. Setting Reaction [Ag3Sn + Cu3Sn] + ε γ Hg → Ag2Hg3 + Cu6Sn5+ Unreacted Ag Sn + voids 3 Cu6Sn5 γ 1 η γ Cu3Sn Ag2Hg3 (γ 1) Ag3Sn Strength Voids Corrosion resistance Creep Dr. Hala Bahgat Dr. Dina Mostafa
- 28. Cu6Sn5 (η) Ag2Hg3 (γ 1) Ag3Sn Voids Dr. Hala Bahgat Dr. Dina Mostafa
