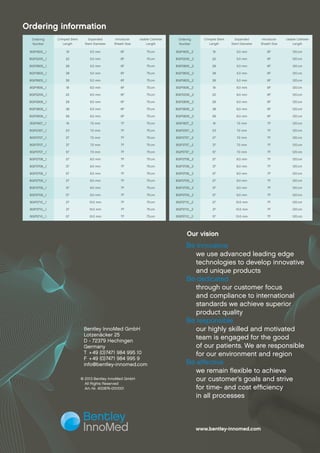
The document contains specifications for peripheral stent graft systems produced by Bentley InnoMed GmbH. It lists ordering numbers, stent lengths and diameters, introducer sheath sizes, and usable catheter lengths for different models. Technical details are provided on the graft material, stent material and design, strut dimensions, introducer compatibility, guidewire compatibility, balloon markers, and pressure ratings. Indications for use and a description of the BeGraft Peripheral system are also included.

![Graft Material
Micro-porous ePTFE
tubing (102 ± 25µm)
Stent Material
(Composition)
CoCr (L605)
Stent Graft Design Single Stent
Strut Dimensions
(Strut Width x Strut Thick-
ness)
0.135 x 0.145 mm (SV)
0.145 x 0.145 mm (MV)
0.165 x 0.145 mm (LV)
Introducer Sheath
Compatibility
6F up to Ø 6 x 58 mm
7F
Guide Wire 0.035”
Shaft Size 5Fr
Balloon Marker
Material
Platinum / Iridium
Nominal Pressure
9 bar Ø 5.0 - 7.0 mm
8 bar Ø 8.0 - 10.0 mm
Rated Burst Pressure
13 bar Ø 5.0 - 7.0 mm
12 bar Ø 8.0 - 10.0 mm
Catheter Shaft Length 75 and 120 cm
Nominal Stent Diameters
5.0 , 6.0 mm (SV)
7.0 , 8.0 mm (MV)
9.0 , 10.0 mm (LV)
Nominal Stent Lengths
18, 22, 28, 38, 58 (SV)
18, 23, 27, 37, 57 (MV)
27, 37, 57 (LV)
Shelf Life 3 years
PERIPHERAL
Peripheral Stent Graft System
PeripheralStentGraftSystem
The Stent Compliance of the BeGraft Peripheral
ADULT
PeripheralInterventions
Your benefits
smart
through a patented ‘one layer’
stent graft solution
competitive
we are proud to have the
lowest profile on the market *
flexible
6F Introducer sheath
compatibility up to ø 6x58 mm
efficient
we have proven excellency
of crossability and trackability *
compatible
biocompatible CoCr alloy
and micro-porous ePTFE
covered
expansion range from
Ø 5.0 mm to Ø 10.0 mm,
stent lengths from 18 mm
to 58 mm
Your specifications
* Data on file at Bentley InnoMed
Indications
The BeGraft Peripheral Stent Graft System is indicated
for intraluminal chronic placement in iliac and
renal arteries for:
Restoring and improving the patency
Treating aneurysms, acute perforations, acute ruptures
and fistulas
Your new Be - formation
Bentley InnoMed is proud to introduce a new arrival
for minimally invasive procedures.
Inflation
Pressure
[bar]
Stent Outer Diameter [mm]
Ø 5.0 Ø 6.0 Ø 7.0 Ø 8.0 Ø 9.0 Ø 10.0
8 8.0 9.0 10.0
9 5.0 6.0 7.0 8.3 9.2 10.2
10 5.2 6.2 7.2 8.5 9.4 10.4
11 5.3 6.4 7.3 8.7 9.6 10.6
12 5.4 6.5 7.5 8.8 9.7 10.7
13 5.5 6.6 7.6
NP Nominal Pressure
Rated Burst PressureRBP
Dear Customer,
Bentley InnoMed guarantees a unique know-
ledge in the development of minimally invasive
products based on many years of hard work
and collaboration with our customers and clients.
As a result of requests from the market,
we combine all our expertise to develop
new products of high quality like the BeGraft
Peripheral Stent System.
This folder has now been enriched with Layar,
which allows you to view digital content with your
smartphone. Dowload the free Layar app, hold
the phone above the images and ‘Tap to View.’
Thank you for your trust,
Miko Obradovic and team
Join innovation by scanning
the new BeGraft Peripheral product
with the Layar app.](https://image.slidesharecdn.com/bgraft-140507052810-phpapp01/85/Bgraft-Peripherial-2-320.jpg)
![Graft Material
Micro-porous ePTFE
tubing (102 ± 25µm)
Stent Material
(Composition)
CoCr (L605)
Stent Graft Design Single Stent
Strut Dimensions
(Strut Width x Strut Thick-
ness)
0.135 x 0.145 mm (SV)
0.145 x 0.145 mm (MV)
0.165 x 0.145 mm (LV)
Introducer Sheath
Compatibility
6F up to Ø 6 x 58 mm
7F
Guide Wire 0.035”
Shaft Size 5Fr
Balloon Marker
Material
Platinum / Iridium
Nominal Pressure
9 bar Ø 5.0 - 7.0 mm
8 bar Ø 8.0 - 10.0 mm
Rated Burst Pressure
13 bar Ø 5.0 - 7.0 mm
12 bar Ø 8.0 - 10.0 mm
Catheter Shaft Length 75 and 120 cm
Nominal Stent Diameters
5.0 , 6.0 mm (SV)
7.0 , 8.0 mm (MV)
9.0 , 10.0 mm (LV)
Nominal Stent Lengths
18, 22, 28, 38, 58 (SV)
18, 23, 27, 37, 57 (MV)
27, 37, 57 (LV)
Shelf Life 3 years
PERIPHERAL
Peripheral Stent Graft System
PeripheralStentGraftSystem
The Stent Compliance of the BeGraft Peripheral
ADULT
PeripheralInterventions
Your benefits
smart
through a patented ‘one layer’
stent graft solution
competitive
we are proud to have the
lowest profile on the market *
flexible
6F Introducer sheath
compatibility up to ø 6x58 mm
efficient
we have proven excellency
of crossability and trackability *
compatible
biocompatible CoCr alloy
and micro-porous ePTFE
covered
expansion range from
Ø 5.0 mm to Ø 10.0 mm,
stent lengths from 18 mm
to 58 mm
Your specifications
* Data on file at Bentley InnoMed
Indications
The BeGraft Peripheral Stent Graft System is indicated
for intraluminal chronic placement in iliac and
renal arteries for:
Restoring and improving the patency
Treating aneurysms, acute perforations, acute ruptures
and fistulas
Your new Be - formation
Bentley InnoMed is proud to introduce a new arrival
for minimally invasive procedures.
Inflation
Pressure
[bar]
Stent Outer Diameter [mm]
Ø 5.0 Ø 6.0 Ø 7.0 Ø 8.0 Ø 9.0 Ø 10.0
8 8.0 9.0 10.0
9 5.0 6.0 7.0 8.3 9.2 10.2
10 5.2 6.2 7.2 8.5 9.4 10.4
11 5.3 6.4 7.3 8.7 9.6 10.6
12 5.4 6.5 7.5 8.8 9.7 10.7
13 5.5 6.6 7.6
NP Nominal Pressure
Rated Burst PressureRBP
Dear Customer,
Bentley InnoMed guarantees a unique know-
ledge in the development of minimally invasive
products based on many years of hard work
and collaboration with our customers and clients.
As a result of requests from the market,
we combine all our expertise to develop
new products of high quality like the BeGraft
Peripheral Stent System.
This folder has now been enriched with Layar,
which allows you to view digital content with your
smartphone. Dowload the free Layar app, hold
the phone above the images and ‘Tap to View.’
Thank you for your trust,
Miko Obradovic and team
Join innovation by scanning
the new BeGraft Peripheral product
with the Layar app.](https://image.slidesharecdn.com/bgraft-140507052810-phpapp01/85/Bgraft-Peripherial-3-320.jpg)