10 152-2-14-2011.
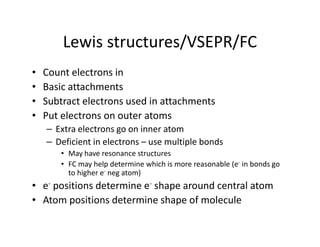
- 1. Lewis structures/VSEPR/FC • Count electrons in • Basic attachments • Subtract electrons used in attachments • Put electrons on outer atoms – Extra electrons go on inner atom – Deficient in electrons – use multiple bonds • May have resonance structures • FC may help determine which is more reasonable (e- in bonds go to higher e- neg atom) • e- positions determine e- shape around central atom • Atom positions determine shape of molecule
- 2. Names of shapes: Electron positions – “lone” (unshared pairs) prefer “more room” Especially important in trig bipyramidal and octahedral
- 5. Bond angles – determine after placing ALL electrons C – 4 bonds, N and O total of 4 pr electrons
- 6. Bond angle Shape of electrons Shape of atoms connections
- 7. d- orbitals will be used in expanded octets
- 8. hybridization • VSEPR predicts bond angles but in 1930’s Linus Pauling used atomic orbitals to to predict these same bond angles – Valence bond theory hybridized orbitals – Atomic orbitals do not have these shapes • Hybridized orbitals – belong to molecules
- 9. When atoms form molecules • Electrons are more “directional”
- 10. C 1s 2s 2p 1s 2s 2p _ _ ___ _ _ ___ Explanation for bond angles seen in molecules
- 12. Hybridization • s, p, d shapes fit the same shapes and bond angles as VSEPR Add to your chart Fig 7.4 Pg 177 fig 7.5
- 13. CN Shape (VSEPR) hybridization 2 Linear sp 3 Trigonal planar sp2 4 Tetrahedral sp3 5 Trigonal bipyramid sp3d 6 octahedral sp3d2
- 14. Add hybridization Bond angle Shape of electrons Shape of atoms connections Note: electrons not added Are all electrons added?
- 15. σ and π bonding • Single bonds – sigma – end to end overlap of orbitals – electron density between nuclei • Multiple bonds – 1 sigma and 1or 2 pi bonds • Pi bonds – side to side overlap – electron density above and below nuclei • Pi bonds weaker than sigmas – Therefore double bond less than twice as strong as single bond
- 16. C2 H4 FIX FOR SP2
- 17. Acetylene – 1 sigma, 2 pi’s C2 H2
- 19. Pi bonds • Overlap of unhybridized p orbitals • C2H6 C2H4 C2H2
- 21. Delocalized electrons • Pi orbitals can be delocalized over several atoms • Alternating =‘s
- 22. Place H’s and e-’s Box sp3 Triangle sp2 Circle atoms with delocalized pi electrons O H3C NH NH O
- 23. CH3 CH3 8 H3C CH3 9 7 H OH 12 10 NH 13 11 5 6 H CH3 14 16 4 15 3 HN 2 1 NH2 HO N HO H N HO H NH N HO H H H H3C N N O NH H3C N O